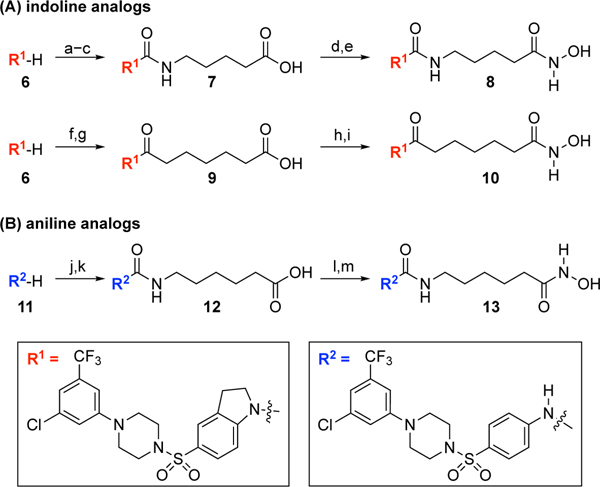
Scheme 1.
The synthesis of indoline and aniline analogs.a
aReagents and conditions: (a) triphosgene, aq. NaHCO3, CH2Cl2, 25 °C, 1 h; (b) NH2(CH2)4CO2Et, DIPEA, CH2Cl2, 25 °C, 1 h, 53% for 2 steps; (c) 1 N NaOH, THF/MeOH (2/1), 25 °C, 30 min, 73%; (d) ClCO2Et, Et3N, THF, 25 °C, 1.5 h; NH2OTBS, MeOH, 25 °C, 1 h, 88%; (e) TFA, CH2Cl2, 25 °C, 25 min, 78%; (f) ClCOCH2(CH2)4CO2Et, Et3N, CH2Cl2, 25 °C, 20 min, 67%; (g) 1 N LiOH, THF/MeOH (2/1), 25 °C, 1.5 h, 99%; (h) ClCO2Et, Et3N, THF, 25 °C, 1 h; NH2OTBS, MeOH, 25 °C, 1 h; (i) TFA, CH2Cl2, 25 °C, 25 min, 21% for 2 steps; (j) OCN(CH2)5CO2Et, CH2Cl2/MeCN (1/1), 25 °C, 18 h, 35%; (k) 1 N NaOH, THF/MeOH (2/1), 25 °C, 3 h, 93%; (l) ClCO2Et, Et3N, THF, 25 °C, 1 h; NH2OTBS, MeOH, 25 °C, 1 h, 94%; (m) TFA, CH2Cl2, 25 °C, 25 min, 81%.