Abstract
Background
The skeletal muscle of pigs is vulnerable to oxidative damage, resulting in growth retardation. Selenoproteins are important components of antioxidant systems for animals, which are generally regulated by dietary selenium (Se) level. Here, we developed the dietary oxidative stress (DOS)-inducing pig model to investigate the protective effects of selenoproteins on DOS-induced skeletal muscle growth retardation.
Results
Dietary oxidative stress caused porcine skeletal muscle oxidative damage and growth retardation, which is accompanied by mitochondrial dysfunction, endoplasmic reticulum (ER) stress, and protein and lipid metabolism disorders. Supplementation with Se (0.3, 0.6 or 0.9 mg Se/kg) in form of hydroxy selenomethionine (OH-SeMet) linearly increased muscular Se deposition and exhibited protective effects via regulating the expression of selenotranscriptome and key selenoproteins, which was mainly reflected in lower ROS levels and higher antioxidant capacity in skeletal muscle, and the mitigation of mitochondrial dysfunction and ER stress. What's more, selenoproteins inhibited DOS induced protein and lipid degradation and improved protein and lipid biosynthesis via regulating AKT/mTOR/S6K1 and AMPK/SREBP-1 signalling pathways in skeletal muscle. However, several parameters such as the activity of GSH-Px and T-SOD, the protein abundance of JNK2, CLPP, SELENOS and SELENOF did not show dose-dependent changes. Notably, several key selenoproteins such as MSRB1, SELENOW, SELENOM, SELENON and SELENOS play the unique roles during this protection.
Conclusions
Increased expression of selenoproteins by dietary OH-SeMet could synergistically alleviate mitochondrial dysfunction and ER stress, recover protein and lipid biosynthesis, thus alleviate skeletal muscle growth retardation. Our study provides preventive measure for OS-dependent skeletal muscle retardation in livestock husbandry.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40104-023-00877-6.
Keywords: Dietary oxidative stress, Endoplasmic reticulum stress, Growth retardation, Mitochondrial dysfunction, Selenoproteins, Skeletal muscle
Background
Skeletal muscle is the largest organ of pigs, which plays a crucial role in the body shape maintenance and body mobility. Skeletal muscle is also the main product that pigs provide for humans. Normal growth of skeletal muscle is accompanied by the deposition of large amounts of proteins and small amounts of lipids. Mammals are susceptible to oxidative stress (OS) after birth, resulting in skeletal muscle growth retardation, which is usually associated with imbalance of protein and lipid metabolism [1]. Current evidence show that OS induces mitochondrial dysfunction and produces excessive reactive oxygen species (ROS), leading to oxidative damage to skeletal muscle, which is accompanied by decreased protein synthesis and increased protein degradation [2]. Mitochondrial dysfunction impairs the respiratory process of adenosine triphosphate (ATP) synthesis [3], the low levels of ATP cannot provide sufficient energy for protein biosynthesis. Besides, excessive ROS inhibits protein synthesis though regulating the mammalian target of rapamycin (mTOR) pathway [4]. Our previous study found that OS promotes lipolysis [5]. Excessive mitochondrial ROS promotes adenosine monophosphate activated protein kinase (AMPK) phosphorylation and inhibits the expression of sterol regulatory element-binding protein 1 (SREBP-1), thus suppress the fatty acid biosynthesis [6]. Hence, mitochondrial dysfunction may be a major factor that mediates the OS-induced skeletal muscle growth retardation.
The endoplasmic reticulum (ER) provides support for protein and lipid biosynthesis and transportation [7]. Under OS, excessive ROS causes ER stress [8], thus impairing protein and lipid biosynthesis [5, 9]. The ER unfolded protein response (ER-UPR) appears to be the representative biomarker of ER stress [10]. Accumulating evidences posit that three ER-localized transmembrane sensors including protein kinase-like ER kinase (PERK), inositol-requiring protein 1 (IRE1), and activating transcription factor 6 (ATF6) mediate the ER-UPR [11–13]. These sensors promote the occurrence of UPR by activating downstream signalling molecules such as eukaryotic translation initiation factor 2 alpha (eIF2α), activating transcription factor 4 (ATF4), C/EBP homologous protein (CHOP), X-box-binding protein 1 (XBP-1), etc. ER-UPR contributes to eliminate excess unfolded or misfolded peptide chains under stress, thus alleviating the ER stress [14]. Thus, it is reasonable to conjecture that mitigation of mitochondrial dysfunction and ER stress may be an effective strategy to alleviate OS-induced skeletal muscle damage and growth retardation.
Dietary supplementation of antioxidants is an effective way to prevent oxidative damage. As a unique and essential trace element for humans and animals, selenium (Se) is the key component of the body's antioxidant system, which can scavenge excess ROS and improve antioxidant capacity [15, 16]. Se exerts its biological functions in mammals predominantly mediated by selenoproteins [17]. Until now, 25 selenoproteins have been found in mammals, among them, 2 of them (GPX4, TXNRD2) are considered as the mitochondria selenoproteins, and 7 of them (DIO2, SELENOF, SELENOK, SELENOM, SELENON, SELENOS and SELENOT) are located in ER [18]. These selenoproteins are characterized by their ability to regulate mitochondrial and ER homeostasis. As an antioxidant enzyme, GPX4 could eliminate intracellular lipid peroxide [19]. SELENON interacts with ryanodine receptors to form the main component of calcium channels, which are involved in the regulation of ER calcium balance and redox reactions [20, 21]. SELENOS removes the misfolded peptide chains and maintains ER homeostasis [22]. Most of the previous studies are focused on the function of a single selenoprotein. Our current studies on the selenotranscriptome reveal that the biological functions of selenoprotein are coordinated by multiple selenoproteins rather than a single [5, 23, 24].
Generally, the expression of selenoproteins in organs of animals is effectively regulated by dietary Se status [25], and Se concentration in muscle is closely corrected with dietary Se levels in form of organic Se [26, 27]. In present study, the new organic Se molecule (hydroxy selenomethionine, OH-SeMet) was used to regulate the expression of selenoproteins. Oxidized diet (formulated with the maize stored over 4 years and oxidized oils) as stress source to determine: 1) the impact of OS on mitochondrial function, ER homeostasis, and protein and lipid metabolism in skeletal muscle of pigs; 2) the potential mechanism linked to the protective effects of Se and selenoproteins.
Materials and methods
Animal, diet and experimental design
The physical properties of the pigs and diets were reported in our previous study [5]. Briefly, 40 crossbreed castrated boars (Duroc × Landrace × Yorkshire) with average body weight of 25.0 ± 3.0 kg were randomly allotted into five dietary treatment groups (n = 8), then, these pigs were fed on basal diet (using normal corn and normal oil, without additional Se supplementation, formulated in accordance with NRC 2012, details in Additional file 1: Table S1) for 7 d to adjust the physiological state and balance the body Se level (based on the biological half-life of Se in piglets [28]). After 7 d, the animals were fed on basal diet (control group, CON) or oxidized diet (the normal corn and oil in basal diet were replaced by aged corn stored over 4 years and oxidized oil) supplied with 0.0 (dietary oxidative stress group, DOS), 0.3 (DOS + 0.3 Se), 0.6 (DOS + 0.6 Se) and 0.9 mg Se/kg (DOS + 0.9 Se) in the form of OH-SeMet for 16 weeks. OH-SeMet (hydroxy selenomethionine, Selisso® Adisseo France S.A.S., Paris, France) is kindly provided by Dr. Kevin Liu and Mr. Allen Gao. The determined dietary Se concentration was shown in Additional file 2: Fig. S1. The pigs were penned in fattening circle house with free access to diet and water, and the house temperature was maintained at 25 ± 2 °C and 20 ± 2 °C for 25−50 kg and > 50 kg stages, respectively. The preparation method of oxidized oils was reported in the previous study [29]. The oxidation characteristics of the diets were shown in Additional file 1: Table S2.
Growth performance
The pigs were weighed every four weeks, after the trial, the average daily gain (ADG) was calculated as follows: ADG = body gain (kg)/test days.
Feedstuff and blood sample collection
Diets were formulated for four times during the animal experiment. After stored at room temperature (20−30 °C) for two weeks, each batch of diet samples (200 g) from different treatment group were collected and kept at −20 °C for lab analysis. At the beginning (0 d) and the end (16 weeks) of the trial, blood samples were collected in sterile vacutainer tubes from the jugular vein and maintained at room temperature for 1 h, centrifuged (3,000 × g) for 10 min, and the serum samples were pipetted into sterile centrifuge tubes, then stored at −20 °C for lab analysis.
Carcass analysis and longissimus dorsi sample collection
At the end of the experiment, a total of thirty pigs (six pigs in each group with a body weight closed to the average body weight) were selected and slaughtered after an overnight fast and sedated by electrical stunning. The carcass weight was estimated as the weight of the hot eviscerated carcass, which was calculated by carcass weight at 45 min after harvest multiplied by 0.98 [30]. The length of the carcass was measured by using a flexible tape on the hanging right half of the carcass at 45 min postmortem, which was the straight distance from the midpoint of the pubic symphysis to the midpoint of the first cervical vertebra. The cross-sectional area of longissimus dorsi (LD) at the 12th rib was confirmed: loin-eye area (cm2) = loin muscle height (cm) × loin muscle width (cm) × 0.7. Tissue samples of LD were dissected between 12th and 13th ribs and snap-frozen in liquid nitrogen, and stored at − 80 °C.
Oxidation characteristics of the diet
After diet samples from different batches were mixed, the acid value (AV), peroxide value (POV), iodine value (IV) and saponification value (SV) in diets of each treatment group were determined by using chemical analysis according to the standards (GB 5009.227–2016, GB 5009.229–2016, GB/T 5532–2008 and GB/T 5534–2008) [31–34].
Selenium concentration in diet, serum and longissimus dorsi
Selenium concentration in diets, serum and LD were measured by using the hybrid generation-atomic fluorescence spectrometer (AFS-230E, Beijing Haiguang instrument, China). After diet samples from different batches were mixed, the diets Se concentration of each treatment group were measured based on the standard (GB/T 13883–2008) [35]. Selenium concentration in serum and LD were measured according to the standard (GB 5009.93–2010) [36]. Samples pretreatment have been described previously [26].
Antioxidant and enzyme analyses
The total glutathione peroxidase (GSH-Px), total antioxidant capability (T-AOC), total superoxide dismutase (T-SOD) and malondialdehyde (MDA) in LD were measured by using the corresponding assay kits (no. A005, A015-1, A001-1–1, A003-1, Nanjing Jiancheng Bioengineering Institute, China). E3 ubiquitin protein ligase (UBE3) and adipose triglyceride lipase (ATGL) in LD were measured by using the commercial enzyme-linked immunosorbent assay (ELISA) kit (no. MM-7800801, MM-220901, Meimian, Jiangsu, China). The concentration of proteins in each sample was determined with the bicinchoninic acid (BCA) method by using a commercial assay kit (no. A045-3, Nanjing Jiancheng Bioengineering Institute). The optical density values were measured with an ultraviolet–visible spectrophotometer (Model 680, Bio-Rad, Hercules, CA, USA).
Measurement of ATP and ROS levels in longissimus dorsi
The ATP levels in LD samples were determined by using a commercial assay kit (no. A095-1–1, Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions. The concentration of ROS in LD samples was measured using an ELISA kit (no. MM-121201, Meimian, Jiangsu, China) according to the manufacturer’s instructions.
Q-PCR analyses of mRNA abundance
The total RNA in each LD sample was extracted by using RNAiso Plus (no. 9109, Takara, Dalian, China) and cDNA was synthesized with the PrimeScript RT reagent kit (no. RR047A, Takara, Dalian, China). The qPCR was performed in a final volume of 10 μL using SYBR Premix Ex TaqTM II kit (no. RR820A, Takara, Dalian, China) on the QuantStudio 6 Flex system (Applied Biosystems, CA, USA). The relative mRNA expression was normalized to the expression of β-Actin and calculated by using the 2−ΔΔCt method as we used previous [37]. The primers for 22 selenogenes, 8 ER stress biomarkers, 4 ubiquitin-related enzymes, 2 lipid synthesis-related genes and β-Actin were designed using Primer Express 3.0 (Applied Biosystems, CA, USA), and listed in Additional file 1: Table S3.
Western blot analyses
Western blotting process was performed as described previously [23]. In brief, each LD sample was homogenized in ice-cold RIPA buffer with PMSF protease inhibitor buffer (Beyotime, Shanghai, China) and centrifuged (12,000 × g) for 30 min at 4 °C. Then, the supernatant of each sample was collected and the total protein concentration was measured using the BCA kit (no. A045-3, Nanjing Jiancheng Bioengineering Institute, China). Protein samples (20 μg) were separated by using 8%−12% SDS-PAGE gels and transferred onto polyvinylidene difluoride membranes (Bio-Rad, CA, USA). The above membranes were blocked with 5% defatted milk for 2 h and washed three times with TBST (prepared with Tris–HCl buffer and isotonic salt solution and contains 1% Tween 20) and incubated overnight at 4 °C with primary antibodies (detailed in Additional file 1: Table S4). Then, these membranes were incubated with corresponding secondary antibodies (anti-rabbit or anti-mouse IgG, 1:5,000, Proteintech Group, IL, USA). The bands were visualized by using an enhanced chemiluminescence system (Bio-Rad, CA, USA), and the densitometric of Western blot bands were analyzed using the Image Lab™ software system (Bio-Rad, Hercules, CA, USA).
Statistical analysis
This study was followed the complete random design (CRD) and applied the one-way structure treatment design. All data are expressed as means ± standard errors. Statistical analyses were performed with the SPSS 27.0 (SPSS Inc., Chicago, USA), values were analyzed using one-way ANOVA followed by Tukey’s multiple range tests, and ANOVA P-values of less than 0.05 were considered statistically significant. Principal component analysis of selenotranscriptome in LD and correlation analysis between the key selenoproteins and other indicators were accomplished by SPSS 27.0 (SPSS, Inc., Chicago, USA). All results were plotted using GraphPad Prism Version 8 software (Graphpad software, LLC, San Diego, USA).
Results
Selenium concentration in serum and longissimus dorsi
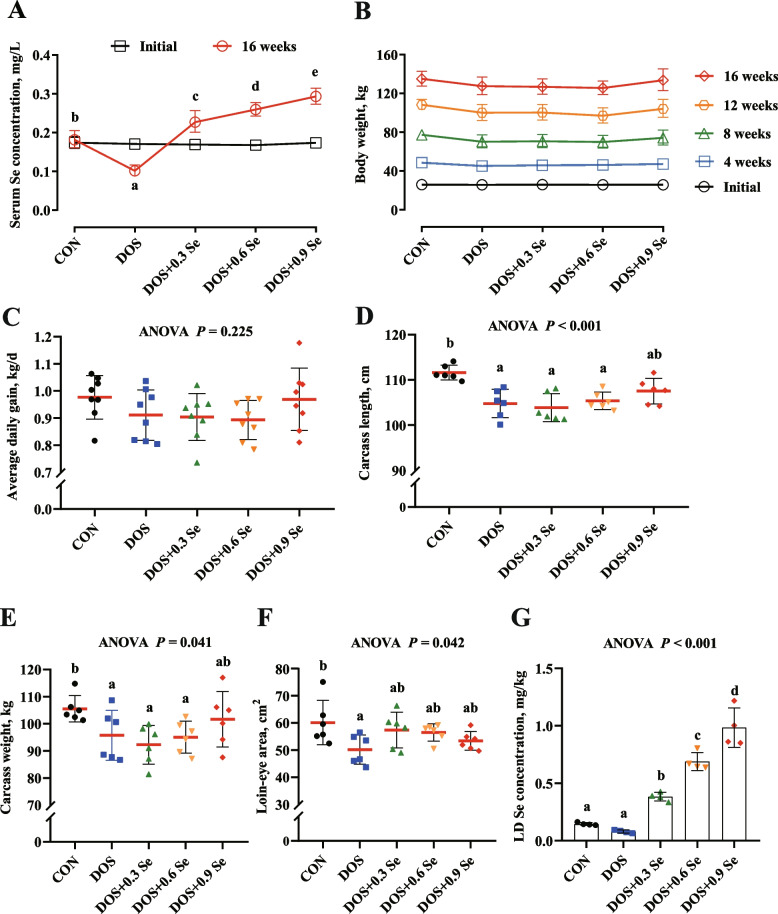
At the beginning of the trial, pigs in each treatment were in the similar serum Se status (0 d) (Fig. 1A). After 16 weeks of treatment, dietary oxidative stress (DOS) decreased (P < 0.05) the Se level in serum (Fig. 1A) and reduced Se deposition in LD (Fig. 1G). Dietary supplementation of OH-SeMet linearly increased (P < 0.05) Se concentrations in serum and LD (Fig. 1A, G).
Fig. 1.
Effects of DOS and OH-SeMet supplementation on serum Se level, growth performance, carcass traits, LD cross-sectional area and LD Se concentration of pigs. A Serum Se concentration; B Body weight; C Average daily gain; D Carcass length; E Carcass weight; F Loin-eye area; G LD Se concentration. Results were expressed as mean ± SD (n = 8 for performance, 6 for carcass traits and 4 for Se concentration), different letters indicate significant differences (P < 0.05)
Growth performance, carcass traits and longissimus dorsi cross-sectional area
To determine the effect of DOS and OH-SeMet supplementation on growth performance of pigs, we recorded the body weight at different stages and measured the carcass traits and the cross-sectional area of LD. As shown in Fig. 1C and B, although there were no statistically significant differences, DOS numerically reduced the ADG and body weight at different stages. Dietary supplementation of 0.9 mg Se/kg OH-SeMet alleviated the negative effects of DOS on growth performance. Slaughter results showed that DOS lowered (P < 0.05) the carcass length, carcass weight and loin-eye area of pigs (Fig. 1D, E and F). While, Se supplementation especially 0.9 mg Se/kg improved the carcass traits and the loin-eye area of pigs faced with DOS.
Antioxidant capacity of longissimus dorsi
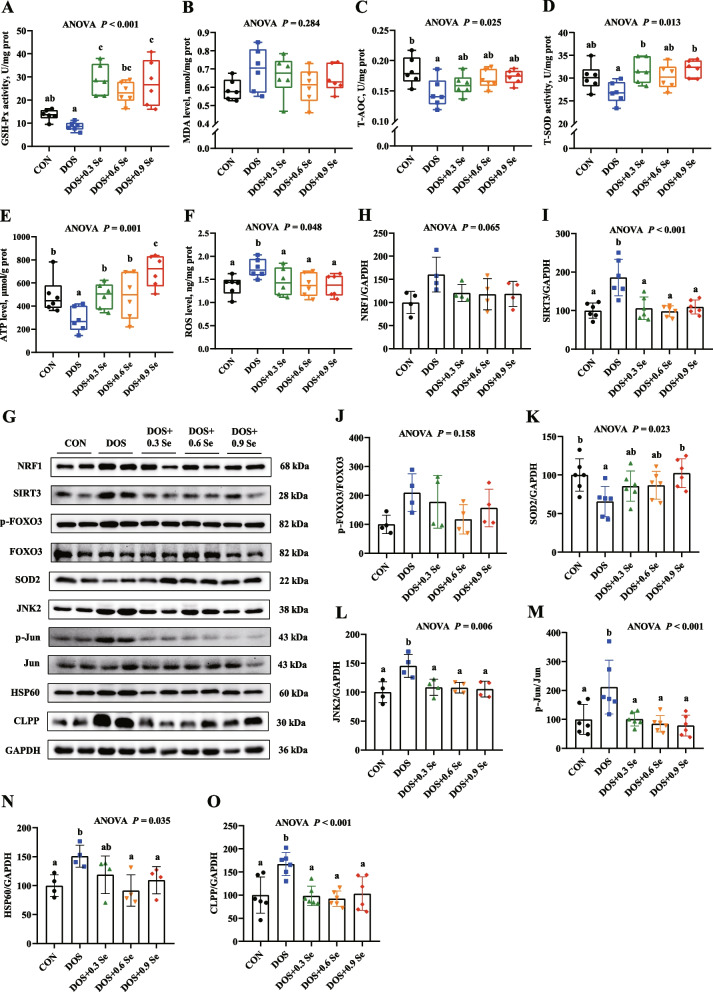
We measured the antioxidant capacity of LD to evaluate whether the prolonged DOS causes oxidative damage to skeletal muscle and the protective effects of Se. Results showed that DOS decreased the antioxidant capacity in LD, these changes were accompanied by the lower activities of GSH-Px, T-AOC and T-SOD (Fig. 2A, C and D). Dietary Se supplementation exhibited protective effects, as reflected by the increased or recovered (P < 0.05) activities of GSH-Px, T-AOC and T-SOD in LD. While, these changes were not dose-dependent with the OH-SeMet levels, except T-AOC.
Fig. 2.
Effects of DOS and OH-SeMet supplementation on antioxidant variables, ATP levels, ROS levels and mitochondrial stress biomarkers in LD. A GSH-Px activity; B MDA levels; C T-AOC activity; D T-SOD activity; E ATP levels; F ROS levels; G Protein expression of mitochondrial stress biomarkers; H Relative protein abundance of NRF1; I Relative protein abundance of SIRT3; J Relative protein abundance of p-FOXO3; K Relative protein abundance of SOD2; L Relative protein abundance of JNK2; M Relative protein abundance of p-Jun; N Relative protein abundance of HSP60; O Relative protein abundance of CLPP. Results were expressed as mean ± SD (n = 6 or 4), different letters indicate significant differences (P < 0.05)
ATP and ROS levels in longissimus dorsi
To evaluate whether DOS induces mitochondria damage and the protective effects of Se, we determined the ATP and ROS levels in LD. Compared with the CON group, pigs faced with DOS showed a lower ATP level and a higher ROS level in LD (Fig. 2E and F). Dietary Se supplementation relieved the negative effect of DOS on the levels of ATP and ROS, which linearly increased (P < 0.05) the ATP levels and decreased (P < 0.05) the ROS levels.
Expression of mitochondrial unfolded protein response biomarkers in longissimus dorsi
The biomarkers of mitochondrial unfolded protein response (MT-UPR) in LD were determined (Fig. 2G). Relative to the CON group, DOS decreased (P < 0.05) the protein level of SOD2 (Fig. 2K), while increased (P < 0.05) the protein levels of NAD-dependent protein deacetylase sirtuin 3 (SIRT3), c-Jun N-terminal kinase 2 (JNK2), phosphorylated transcription factor AP1 (p-Jun), heat shock protein 60 (HSP60) and caseinolytic mitochondrial matrix peptidase (CLPP) (Fig. 2I, L, M, N and O). Besides, DOS tended to increase (0.05 < P < 0.1) the level of nuclear respiratory factor 1 (NRF1) (Fig. 2H). Dietary Se supplementation exhibited protective effects, which were reflected in the increased protein level of SOD2 and the decreased (P < 0.05) protein abundance of SIRT3, CLPP, JNK2, p-Jun, HSP60 and NRF1. However, these changes were not dose-dependent with the OH-SeMet levels. Besides, DOS and Se supplementation showed no effects (P > 0.05) on the protein abundances of p-FOXO3.
Expression of ER stress biomarkers in longissimus dorsi
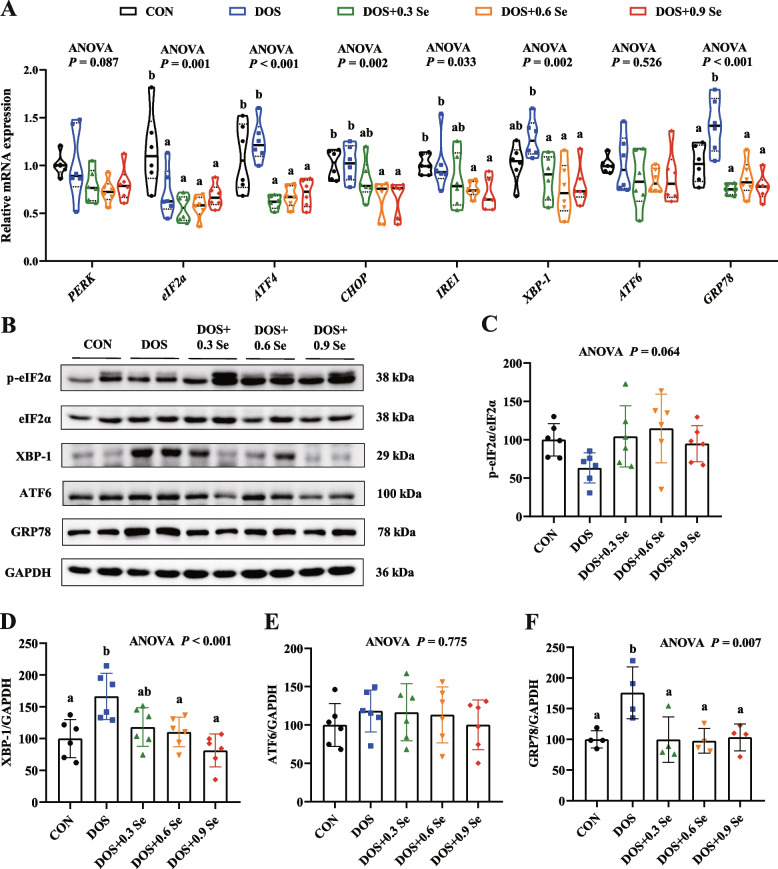
We further determined the ER stress biomarkers in LD (Fig. 3). Firstly, we determined the mRNA expression of 8 ER-UPR markers (Fig. 3A). Compared with the CON group, DOS down-regulated (P < 0.05) the mRNA expression of eIF2α, up-regulated (P < 0.05) GRP78 level, and tended to increase (0.05 < P < 0.1) XBP-1 level. Dietary Se supplementation decreased (P < 0.05) the mRNA abundance of ATF4, CHOP, IRE1, XBP-1 and GRP78. DOS and Se supplementation exhibited limited effects (P > 0.05) on the mRNA expression of PERK and ATF6.
Fig. 3.
Effects of DOS and OH-SeMet supplementation on the expression of ER stress biomarkers in LD. A The mRNA expression of PERK, eIF2α, ATF4, CHOP, IRE1, XBP-1, ATF6 and GRP78; B Protein expression of ER stress biomarkers; C Relative protein abundance of p-eIF2α; D Relative protein abundance of XBP-1; E Relative protein abundance of ATF6; F Relative protein abundance of GRP78. Results were expressed as mean ± SD (n = 6 or 4), different letters indicate significant differences (P < 0.05)
We further examined the protein expression of four ER stress biomarkers (Fig. 3B). Results showed that DOS increased (P < 0.05) the protein abundance of XBP-1 and GRP78 (Fig. 3D and F), and tended to decrease (0.05 < P < 0.1) p-eIF2α (Fig. 3C). Dietary Se supplementation recovered (P < 0.05) the protein levels of XBP-1 and GRP78, while tended to increase the protein abundance of p-eIF2α. While, these changes were not dose-dependent with the OH-SeMet levels except XBP-1. Besides, DOS and Se supplementation showed no effects on the protein expression of ATF6 (Fig. 3E).
Protein metabolism indicators in longissimus dorsi
The protein metabolism indicators in LD were determined (Fig. 4). Relative to the CON group, long-term DOS promoted protein degradation in skeletal muscle, which is mainly reflected in the up-regulation of UBA1, UBA2 and UBE2K mRNA levels (Fig. 4A), and the increased activity of UBE3 (Fig. 4B). Besides, DOS decreased the relative protein abundance of p-AKT, p-mTOR and p-S6K1 (Fig. 4E, F, H), thus suppressed the AKT-mTOR-S6K signalling pathway and led to protein biosynthesis inhibition in LD. Dietary Se supplementation reversed the negative impact of DOS. Pigs received additional dietary Se showed the lower UBA1, UBA2 and UBE2K mRNA profiles and UBE3 activity (P < 0.05). Se supplementation also increased (P < 0.05) the protein expression of p-AKT, p-mTOR and p-S6K1 (Fig. 4E, F and H). While, several parameters such as the UBE2B levels, p-AKT and p-mTOR abundances did not showed the dose-dependent relationship with OH-SeMet levels.
Fig. 4.
Effects of DOS and OH-SeMet supplementation on the protein metabolism and lipid metabolism in LD. A Relative mRNA expression of UBA1, UBA2, UBE2B, UBE2K, ACACA and FASN; B Activity of UBE3; C Protein expression of AKT-mTOR and AMPK-SREBP-1 signalling pathway; D Activity of ATGL; E Relative protein abundance of p-AKT; F Relative protein abundance of p-mTOR; G Relative protein abundance of p-4E-BP1; H Relative protein abundance of p-S6K1; I Relative protein abundance of p-AMPKα; J Relative protein abundance of SREBP-1. Results were expressed as mean ± SD (n = 6 or 4), different letters indicate significant differences (P < 0.05)
Lipid metabolism indicators in longissimus dorsi
We further investigated the lipid metabolism indicators in LD of pigs (Fig. 4). DOS affected the AMPK-SREBP-1 signalling pathway, which is mainly reflected in the up-regulation (P < 0.05) of p-AMPKα protein level (Fig. 4I) and down-regulation (P < 0.05) of SREBP-1 protein level (Fig. 4J) and mRNA expression of FASN (Fig. 4A), thus suppressed the lipid biosynthesis in LD. Besides, DOS increased (0.05 < P < 0.1) the activity of ATGL (Fig. 4D). Dietary Se supplementation showed positive impacts on the lipid metabolism indicators in LD under DOS. OH-SeMet supplementation recovered (P < 0.05) the protein levels of p-AMPKα, increased (P < 0.05) the protein expression of SREBP-1, up-regulated (P < 0.05) the mRNA levels of ACACA and FASN. Besides, 0.6 and 0.9 mg Se/kg OH-SeMet decreased (P < 0.05) the activity of ATGL. While, several indicators such as ACACA levels, ATGL activity and p-AMPKα abundance did not showed the dose-dependent relationship with OH-SeMet levels.
Expression of selenotranscriptome in longissimus dorsi
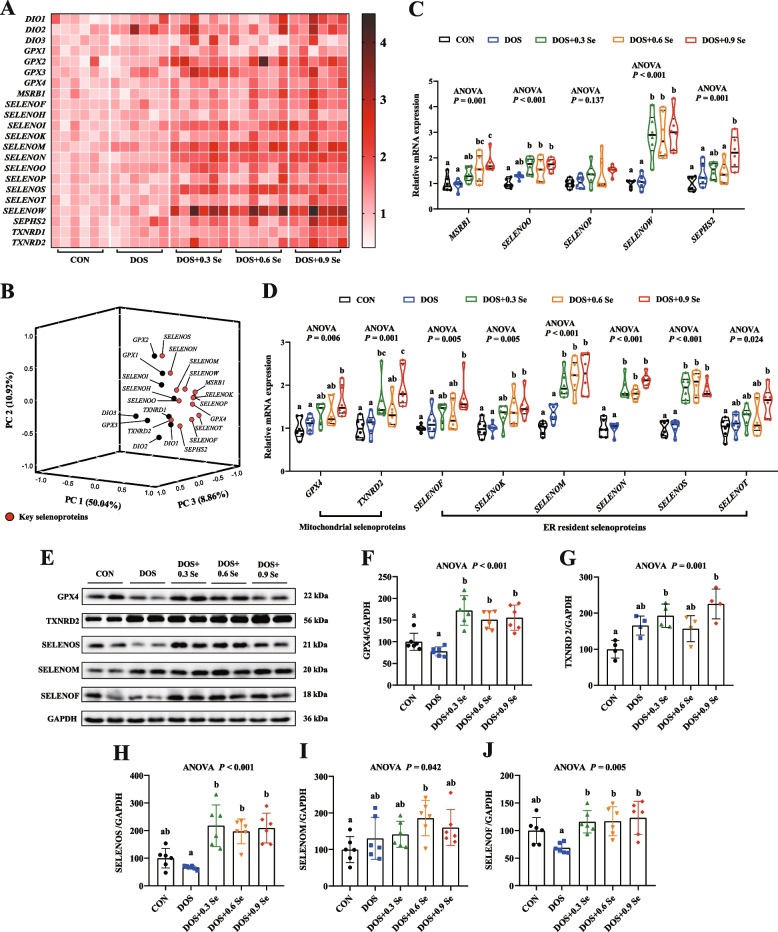
Se exerts its most-known biological functions mainly through selenoproteins. Here, the selenotranscriptome in LD of pigs were determined. GPX6, SELENOV and TXNRD3 in skeletal muscle were in poor quality or low expression and were not reported herein. DOS alone showed limited impact on the expression of these selenogenes, except up-regulated (P < 0.05) the expression of DIO2 and GPX4 (Fig. 5A, detailed profiles are shown in Additional file 3: Fig. S2). Dietary Se supplementation especially 0.9 mg Se/kg increased (P < 0.05) the abundance of 15 selenogenes (DIO1, GPX2, GPX3, MSRB1, SELENOI, SELENOK, SELENOM, SELENON, SELENOO, SELENOS, SELENOT, SELENOW, SEPHS2, TXNRD1 and TXNRD2) in LD of pigs faced with DOS. Besides, DOS and Se supplementation exhibited limited impacts (P > 0.05) on the expression of DIO3, GPX1, SELENOH and SELENOP.
Fig. 5.
Effects of DOS and OH-SeMet supplementation on the expression of selenotranscriptome and 5 key selenoproteins in LD. A Heatmap of mRNA abundance of selenotranscriptome; B Principal component analysis of the selenotranscriptome; C and D Relative mRNA expression of 13 key selenogenes; E Protein expression of 5 key selenoproteins; F Relative protein abundance of GPX4; G Relative protein abundance of Txnrd2; H Relative protein abundance of SelS; I Relative protein abundance of SelM; J Relative protein abundance of SelF. Results were expressed as mean ± SD (n = 6 or 4), different letters indicate significant differences (P < 0.05)
Expression of key selenoproteins in longissimus dorsi
We further performed a principal component analysis through the mathematical method of dimensionality reduction to distinguish the key selenoproteins affected by DOS and OH-SeMet. Three comprehensive variables were chosen to reflect the original variable information (Fig. 5B). Results showed that 13 selenogenes were observed at relatively distant positions in three-dimensional space. Thus, these 13 selenogenes (GPX4, MSRB1, SELENOF, SELENOK, SELENOM, SELENON, SELENOO, SELENOP, SELENOS, SELENOT, SELENOW, SEPHS2, TXNRD2) were the key genes affected by DOS and OH-SeMet. Compared with the DOS group, these key genes showed the higher mRNA levels in Se supplementation groups, except SELENOP (Fig. 5C and D). Among these key genes, 2 of them (GPX4, TXNRD2) are considered as the mitochondria selenoproteins, 6 of them (SELENOF, SELENOK, SELENOM, SELENON, SELENOS, SELENOT) are located in the ER.
Thus, we further determined the protein abundance of GPX4, TXNRD2, SELENOF, SELENOM and SELENOS in LD (Fig. 5E). DOS tended to increase (0.05 < P < 0.1) the relative protein levels of TXNRD2 and SELENOM (Fig. 5G and I), while tended to decrease (0.05 < P < 0.1) the protein levels of SELENOF and SELENOS (Fig. 5J and H). Compared with the DOS group, Se supplementation increased (P < 0.05) the relative protein abundance of GPX4, SELENOF and SELENOS, and tended to increase (0.05 < P < 0.1) the levels of TXNRD2 and SELENOM. Interestingly, the protein abundance of these 5 selenoproteins did not showed the dose-dependent relationship with OH-SeMet levels.
Correlation analysis
Correlation analysis was further conducted to estimate the potential relationship between the key selenogenes and other measures that were changed significantly under DOS and OH-SeMet supplementation (details are provided in Additional file 1: Table S5). As shown in Fig. 6, significant positive correlations were found among most of the 12 key selenogenes. Three genes (SELENOM, SELENON, SELENOS) exhibited the primary negative correlations with the mitochondrial stress biomarkers (ROS, SIRT3, CLPP, JNK2, p-Jun and HSP60), while exhibited the primary positive correlations with the protein synthesis indicators (p-AKT, p-mTOR, p-S6K1). Four genes (SELENOW, SELENOM, SELENON, SELENOS) were negatively correlated with ER stress biomarkers (ATF4, XBP-1, GRP78). Five genes (MSRB1, SELENOW, SELENOM, SELENON, SELENOS) exhibited the primary negative correlations with protein degradation indicators (UBA1, UBA2, UBE2B, UBE2K, UBE3). Almost all the key selenogenes were positively correlated with the antioxidant variables (GSH-Px, T-AOC, T-SOD, SOD2) and ATP. Besides, SELENON and SELENOS were positively correlated with the lipid synthesis indicators (SREBP-1 and ACACA).
Fig. 6.
Correlation analysis between the key selenogenes and other measures. Pearson correlation coefficients were provided in Additional file 1: Table S5. The color of red represents a significant positive correlation (P < 0.05), blue represents a significant negative correlation (P < 0.05)
Discussion
Dietary oxidative stress comprises rancid oil, moldy food, aged grain, etc., which is a common oxidative stressor in animal husbandry, these diets is rich in lipid peroxidation products after heating and/or long-term storage [38, 39], thus cause oxidative damage to animals. Current evidences suggest that lipid peroxidation product intake induces OS and promotes the disease progression and impairs the growth of skeletal muscle [1, 40]. In present study, a long-term DOS was administered to the growing-finishing pigs to assess the negative effect of OS on skeletal muscle growth. Pigs faced with DOS exhibited the numerically lower body weight in each period and the lower ADG, carcass length and carcass weight. Skeletal muscle accounts for more than 50% of a pig's body weight [41], it is reasonable to expect that DOS affects skeletal muscle growth. LD is the key component of porcine skeletal muscle, and its cross-sectional area can used to effectively evaluate the skeletal muscle growth status of pigs [42]. Similarly, DOS decreased the LD cross-sectional area. These results suggest that long-term DOS causes skeletal muscle growth retardation in pigs. Selenium is considered to be an excellent antioxidant, and Se concentration in muscle is closely corrected with dietary Se levels in form of organic Se [26, 27]. In this study OH-SeMet supplementation increased the serum and LD Se concentration in a dose dependent pattern. Likewise, dietary OH-SeMet supplementation especially 0.9 mg Se/kg increased the body weight, carcass length, carcass weight and LD cross-sectional area of pigs under DOS. Therefore, organic Se supplementation in form of OH-SeMet alleviates skeletal muscle growth retardation of pigs suffered with DOS.
Oxidative damage is the major factor that causes skeletal muscle growth retardation. The main cause of oxidative damage is the excessive ROS produced by dysfunctional mitochondria, which exceeds the carrying capacity of the antioxidant system [43]. Mitochondrial dysfunction impairs the oxidative phosphorylation process and affects the ATP biosynthesis [44, 45]. Here, we further evaluated the oxidative damage status in skeletal muscle of pigs faced with DOS and Se. In present study, DOS decreased the antioxidant capacity of LD, which is mainly reflected in the lower activities of total GSH-Px, T-AOC and T-SOD. Besides, DOS decreased the ATP level and increased the ROS level in LD, which suggest that DOS causes mitochondrial dysfunction and induces oxidative damage in skeletal muscle. Interestingly, dietary Se supplementation decreased the ROS levels and increased ATP levels, which is alongside with the improvement of antioxidant capacity in LD.
To explore whether the potential protective effect of Se is associated with alleviation of mitochondrial dysfunction, we measured the expression of MT-UPR biomarkers in LD. MT-UPR is a conserved signalling pathway in mammals and has gained extensive attention as an important regulatory pathway for mitochondrial communication [46]. Under stress conditions, the MT-UPR senses insufficient protein-handling capacity in mitochondria and aims to restore mitochondrial homeostasis by expanding the folding capacity and enhancing protein degradation [47]. There are three distinct branches involved in the MT-UPR signalling pathway in mammals [46, 48]. One pathway is that excessive ROS phosphorylates AKT and regulates the expression of mitochondrial regulator NRF1, thus initiating MT-UPR. Mitochondrial dysfunction also activates SIRT3, which induces deacetylation of FOXO3 into the nucleus and enhances the transcription of the ROS detoxification gene SOD2. Besides, accumulation of unfolded proteins in the mitochondrial matrix can activate transcription factor Jun by JNK2, phosphorylated Jun leading to the induction of CHOP and CCAAT/enhancer-binding protein-β (CEBPβ), thus initiating MT-UPR. Molecular chaperones and proteases in the mitochondrial matrix, such as HSP60, CLPP, constitute major targets of MT-UPR signalling. Enhancing their expression could effectively scavenge unfolded proteins from the mitochondrial matrix, thus maintaining mitochondrial function [49, 50]. In present study, DOS decreased the protein level of SOD2, increased SIRT3, JNK2, p-Jun, HSP60 and CLPP. Dietary Se supplementation recovered the protein abundance of SOD2 and decreased the protein levels of SIRT3, JNK2, p-Jun, HSP60 and CLPP, thus alleviates mitochondrial dysfunction, and this protective process is alongside with regulation of SIRT3/FOXO3/SOD2 and JNK2/Jun/CHOP signalling pathways.
Under OS, excessive ROS produced by dysfunctional mitochondria generally causes ER stress and ER dysfunction, thus affects the biosynthesis of protein and lipid [8, 9]. ER-UPR is the representative behavior of the ER stress [51]. Under ER stress, abundance of unfolded proteins accumulated in the ER facilitate the activation of ER-UPR through three ER transmembrane sensors: PERK, IRE1 and ATF6 [11–13]. These sensors contribute to the restoration of ER homeostasis by regulating multiple downstream signalling molecules such as eIF2α, ATF4, CHOP and XBP-1, and molecular chaperone such as GRP78 is the major target of ER-UPR signalling in the ER lumen [52]. ER stress generally increases the expression of these biomarkers. In this study, DOS increased the abundance of XBP-1 and GRP78 at mRNA and protein levels, and decreased the mRNA expression of eIF2α in LD, indicating that DOS causes ER stress and promotes the occurrence of ER-UPR. Pigs received three levels of OH-SeMet showed the lower ATF4, CHOP, IRE1, XBP-1 and GRP78 mRNA levels, and the lower XBP-1 and GRP78 protein levels in LD. These results ulteriorly reveal that OH-SeMet contributes to improve the ER homeostasis in skeletal muscle under DOS through regulating the ER-UPR, thus improves protein and lipid metabolism homeostasis.
The maintenance of mitochondrial and ER homeostasis provides guarantee for protein and lipid synthesis, and OH-SeMet supplementation effectively alleviates mitochondrial dysfunction and ER stress in skeletal muscle under OS. These results prompted us to further investigate the effects of Se on protein and lipid metabolism in skeletal muscle under DOS. For protein metabolism, DOS increased the mRNA expression of UBA1, UBA2 and UBE2K, and increased the activity of UBE3 in LD. The ubiquitin–proteasome pathway plays a unique role in the degradation of skeletal muscle proteins [53]. In this process, ubiquitin molecules are activated by ubiquitin-activating enzyme (UBE1) and transported to ubiquitin-conjugating enzyme (UBE2), UBE2 and UBE3 cooperate to bind ubiquitin molecules to proteins, thus degrading proteins [54]. UBA1 and UBA2 are common subtypes of UBE1, UBE2B and UBE2K are common subtypes of UBE2. Our results suggest that DOS promotes protein degradation in porcine skeletal muscle. mTOR pathway has gained attention as a key regulator of protein biosynthesis [55]. Phosphorylated mTOR inhibits 4E-BP1 and activates S6K to promote the translation of ribosomal S6 [56, 57]. And mTOR pathway is generally regulated by the upstream signal AKT. Evidence shows that excessive ROS inhibits protein synthesis though regulating the 4E-BP1 and S6K [4]. In present study, DOS suppressed the protein abundance of p-AKT, p-mTOR and p-S6K1, suggesting DOS inhibits protein synthesis in skeletal muscle. Interestingly, dietary OH-SeMet supplementation suppressed protein degradation and promoted protein biosynthesis in skeletal muscle, which were accompanied by the down-regulation of UBA1, UBA2, UBE2B and UBE2K mRNA levels, and the lower activity of UBE3, while the increase of p-AKT, p-mTOR and p-S6K1 protein levels. For lipid metabolism, we found that DOS decreased the mRNA expression of FASN and the protein abundance of SREBP-1, and increased the activity of ATGL and the protein expression of p-AMPK. FAS encoded by FASN is the key enzyme that regulates fatty acid synthesis, which promotes the elongation of fatty acid chains [58]. ACC encoded by ACACA is the rate-limiting enzyme in the process of fatty acid de novo synthesis, similar to FAS, ACC is regulated by nuclear transcription factor SREBP-1 [59, 60]. The activation of SREBP-1 is usually regulated by AMPK. ATGL facilitate the specifically hydrolyzation of the first ester bond of triglyceride, which is considered to be the rate-limiting enzyme in the lipolysis [61]. Our present study confirms that DOS suppresses the lipid biosynthesis and promotes lipolysis in LD of pigs. Dietary Se supplementation exhibited protective effects, which are reflected in the up-regulation of ACACA, FASN mRNA levels and SREBP-1 protein levels, the lower ATGL activity and recovered protein expression of p-AMPK. Based on these results, OH-SeMet supplementation improves protein and lipid metabolism homeostasis in skeletal muscle of pigs under DOS, and this process is medicated with the regulating of AKT/mTOR/S6K1 and AMPK/SREBP-1 pathway.
Our previous studies of the selenotranscriptome in different animal models suggest that Se performs its biological function through the synergy of multiple selenoproteins [5, 23, 26, 62]. Hence, we explored the mRNA expression of selenotranscriptome in the skeletal muscle and identified key selenogenes using principal component analysis. Thirteen among 22 selenogenes were identified as the key genes response to DOS and OH-SeMet supplementation. Among these selenoproteins, GPX4 and TXNRD2 are considered as the mitochondria selenoproteins, SELENOF, SELENOK, SELENOM, SELENON, SELENOS and SELENOT are located in ER, while the other 4 selenoproteins (MSRB1, SELENOP, SELENOW, SEPHS2) are in a free state [18]. At present, the location and function of SELENOO are remain unclear, study suggests that SELENOO located in the mitochondria and is associated with redox control [63]. MSRB1 was found to control the assembly and disassembly of actin in mammals, suggesting it may be involved in skeletal muscle growth regulation [64]. What's more, MSRB1 participates in the regulation of redox homeostasis and protect proteins from oxidative damage [65]. SELENOP is mainly responsible for the transport of Se, which can transport Se from plasma to various target organs, thus controlling the expression of all selenoproteins alongside with SEPHS2 [66]. About the mitochondrial selenoproteins, GPX4 could eliminate intracellular lipid peroxide, TXNRD2 protects against disulfide damage and regulates redox homeostasis, lack of GPX4 and TXNRD2 is lethal in mice [18, 67]. About the ER resident selenoproteins, most of them are involved in the regulation of ER homeostasis. SELENOF participates in the protein quality control by mediating disulfide bond formation, which can clear excess unfolded or misfolded proteins under ER stress conditions [68, 69]. SELENON interacts with ryanodine receptors to form the main component of calcium channels, which is involved in the regulation of ER calcium balance and redox reactions [20, 21]. Besides, previous study found that SELENON affects skeletal muscle development, and knockout of the SELENON causes the destruction of the muscle structure in zebrafish embryonic [70]. SELENOS is the central component of retro-translocation channel in ER-associated protein degradation, which can remove misfolded peptide chains and maintains ER homeostasis [22, 71]. SELENOK, SELENOM and SELENOT control the ER homeostasis though scavenging excessive ROS and suppressing apoptosis [72–75]. In the present study, DOS alone exhibited limited impact on the mRNA expression of these selenoproteins, while tended to increase the protein abundance of TXNRD2 and SELENOM, and tended to decrease the protein levels of SELENOS and SELENOF. Dietary OH-SeMet supplementation up-regulated the expression of these key selenoproteins at mRNA or protein levels, except SELENOP. Based on the above results, it is clear that OH-SeMet alleviates skeletal muscle growth retardation induced by DOS though regulating the expression of 12 key selenogenes.
We further performed correlation analysis to demonstrate the synergistic effect of selenoproteins and the relationship between 12 key selenogenes and these assayed indicators. The significant positive correlations were found among most of the 12 genes, and almost all the genes were positively correlated with the antioxidant variables (GSH-Px, T-AOC, T-SOD, SOD2) and ATP levels, which validates our hypothesis that Se performs its biological function through the synergy of multiple selenoproteins. Interestingly, the results of correlation analysis showed that a subset selenogenes showed significant positive correlation in the process of alleviating mitochondrial dysfunction and ER stress, such as MSRB1, SELENOW, SELENOM, SELENON and SELENOS, and lipid and protein metabolism-related indicators analysis showed the similar results. Based on these results, it is reasonable to believe that dietary OH-SeMet supplementation improves lipid and protein metabolism in skeletal muscle of pigs under DOS mainly through regulating a few key selenoproteins (MSRB1, SELENOW, SELENOM, SELENON and SELENOS), while the other selenoproteins play an auxiliary role in this process.
Conclusion
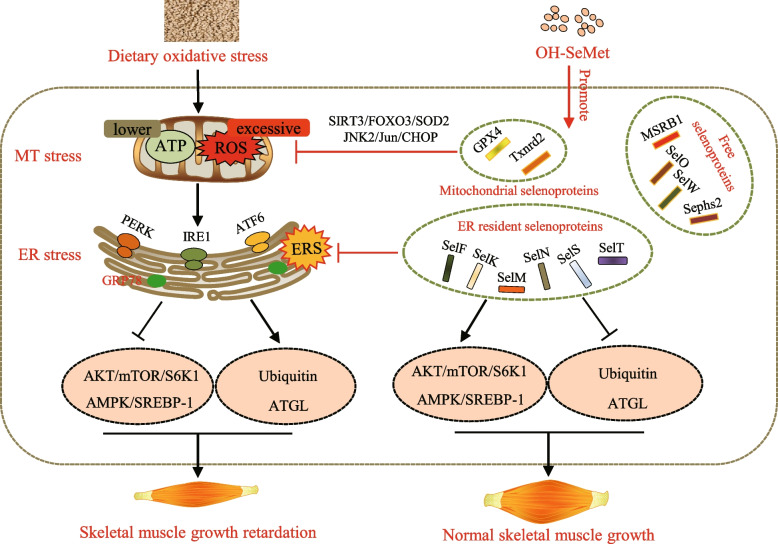
In present study, we reveal that the DOS-induced skeletal muscle retardation in pigs is associated with mitochondrial dysfunction and ER stress, which suppresses the protein and lipid biosynthesis. Dietary supplementation of organic Se in form of OH-SeMet linearly increases the deposition of Se in skeletal muscle, enabling skeletal muscle quickly mobilize Se reserves to synthesize selenoproteins under stress. Multiple in vivo evidences suggest that increased expression of selenoproteins alleviate mitochondrial dysfunction and ER stress, recover protein and lipid biosynthesis, thus alleviate skeletal muscle retardation. Several key selenoproteins exhibit synergistic effects in this process. Our results contribute to better understanding on the synergistic mechanism of key selenoproteins against OS-induced skeletal muscle growth retardation in mammals (Fig. 7), which provide preventive measure for OS-dependent skeletal muscle retardation in livestock husbandry.
Fig. 7.
Schematic diagram of selenoproteins alleviating skeletal muscle growth retardation of pigs induced by DOS. Several key selenoproteins synergistically scavenge excessive ROS, release mitochondrial and ER stress, and restore the inhibition of protein and lipid biosynthesis caused by dietary oxidative stress, thus alleviate skeletal muscle growth retardation
Supplementary Information
Additional file 1: Table S1. Composition and nutrient levels of the basal diet. Table S2. The oxidation Characteristics of the diets. Table S3. Primers used for the Q-PCR. Table S4. Primary antibodies for the Western blot analyses. Table S5. The correlation analysis between the 12 key selenogenes and other measures.
Additional file 2: Fig. S1. Diet Se concentration.
Additional file 3: Fig. S2. The mRNA expression of the 22 selenogenes.
Acknowledgements
We thank Dr. Mickael Briens, Dr. Kevin Liu and Mr. Allen Gao for their technical support.
Abbreviations
- ADG
Average daily gain
- AMPK
Adenosine monophosphate activated protein kinase
- ATF4
Activating transcription factor 4
- ATF6
Activating transcription factor 6
- ATGL
Adipose triglyceride lipase
- ATP
Adenosine triphosphate
- AV
Acid value
- CHOP
C/EBP homologous protein
- CLPP
Caseinolytic mitochondrial matrix peptidase
- DOS
Dietary oxidative stress
- eIF2α
Eukaryotic translation initiation factor 2 alpha
- ER
Endoplasmic reticulum
- ER-UPR
Endoplasmic reticulum unfolded protein response
- FOXO3
Forkhead box O3
- GSH-Px
Glutathione peroxidase
- HSP60
Heat shock protein 60
- IRE1
Inositol-requiring protein 1
- IV
Iodine value
- JNK2
C-Jun N-terminal kinase 2
- Jun
Transcription factor AP1
- LD
Longissimus dorsi
- MDA
Malondialdehyde
- mTOR
Mammalian target of rapamycin
- MT-UPR
Mitochondrial unfolded protein response
- NRF1
Nuclear respiratory factor 1
- OH-SeMet
Hydroxy selenomethionine
- p-AMPKα
Phosphorylated adenosine monophosphate activated protein kinase alpha
- p-eIF2α
Phosphorylated eukaryotic translation initiation factor 2 alpha
- PERK
Protein kinase-like endoplasmic reticulum kinase
- p-FOXO3
Phosphorylated forkhead box O3
- p-Jun
Phosphorylated transcription factor AP1
- POV
Peroxide value
- ROS
Reactive oxygen species
- SIRT3
NAD-dependent protein deacetylase sirtuin 3
- SREBP-1
Sterol regulatory element-binding protein 1
- SV
Saponification value
- T-AOC
Total antioxidant capability
- T-SOD
Total superoxide dismutase
- UBE3
E3 ubiquitin protein ligase
- XBP-1
X-box-binding protein 1
Authors’ contributions
JJ and HZ designed this research. JJ ran all the experiments with help from YH, YL, JT, LW, GJ, GL, XC, GT, JC, LC, BK. JJ and HZ wrote this article. All authors reviewed and revised the article, and approved the final version.
Funding
This work was partially supported by the National Natural Science Foundation of China (No. 31772643 and 31272468), and the Special Research Funding for Discipline Construction in Sichuan Agricultural University (No. 03570126) and Adisseo France (18SES533).
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
The animal trial was approved by the Animal Care and Use Committee of the Laboratory Animal Center at Sichuan Agricultural University (Ethics Approval Code: SCAUAC201904-4).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Jinzhong Jing and Ying He contributed equally to this work.
References
- 1.Kumar A, Davuluri G, Welch N, Kim A, Mahesha M. Oxidative stress mediates ethanol-induced skeletal muscle mitochondrial dysfunction and dysregulated protein synthesis and autophagy. Free Radical Bio Med. 2019;145:284–99. doi: 10.1016/j.freeradbiomed.2019.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shenoy PS, Sen U, Kapoor S, Ranade AV, Chowdhury CR, Bose B. Sodium fluoride induced skeletal muscle changes: degradation of proteins and signaling mechanism. Environ Pollut. 2019;244:534–548. doi: 10.1016/j.envpol.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 3.Baltazar R. Oxidative stress impairs the respiratory process of ATP synthesis. FASEB J. 2007;21(5):A296. [Google Scholar]
- 4.Toshniwal AG, Gupta S, Mandal L, Mandal S. Ros inhibits cell growth by regulating 4EBP and S6K, independent of TOR, during development. Dev Cell. 2019;49(3):473–489. doi: 10.1016/j.devcel.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jing JZ, Yin SG, Liu Y, Liu YG, Wang LQ, Tang JY, et al. Hydroxy selenomethionine alleviates hepatic lipid metabolism disorder of pigs induced by dietary oxidative stress via relieving the endoplasmic reticulum stress. Antioxidants. 2022;11(3):552. doi: 10.3390/antiox11030552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Park J, Han SJ, Park I, Huu TN, Kim JS, et al. The critical role of redox regulation of PTEN and peroxiredoxin III in alcoholic fatty liver. Free Radical Bio Med. 2021;162(5):141–148. doi: 10.1016/j.freeradbiomed.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Baumann O, Walz B. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int Rev Cytol. 2011;205:149–214. doi: 10.1016/S0074-7696(01)05004-5. [DOI] [PubMed] [Google Scholar]
- 8.Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012;19(11):1880–1891. doi: 10.1038/cdd.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths B, Lewis CA, Bensaad K, Ros S, Zhang Q, Ferber EC, et al. Sterol regulatory element binding protein-dependent regulation of lipid synthesis supports cell survival and tumor growth. Cancer Metab. 2013;1(1):3. doi: 10.1186/2049-3002-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemmer IL, Willemsen N, Hilal N, Bartelt A. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol Metab. 2021;47:101169. doi: 10.1016/j.molmet.2021.101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrara M, Prischi F, Nowak PR, Ali MM. Crystal structures reveal transient PERK luminal domain tetramerization in endoplasmic reticulum stress signaling. EMBO J. 2015;34:1589–1600. doi: 10.15252/embj.201489183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calfon M, Zeng HQ, Urano F, Till JH, Hubbard SR, Harding HP, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–6. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 13.Ye J, Rawson RB, Komuro R, Chen X, Davé UP, Prywes P, et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6(6):1355–1364. doi: 10.1016/S1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 14.Sage AT, Holtby-Ottenhof S, Shi Y, Damjanovic S, Sharma AM, Werstuck GH. Metabolic syndrome and acute hyperglycemia are associated with endoplasmic reticulum stress in human mononuclear cells. Obesity. 2012;20:748–755. doi: 10.1038/oby.2011.144. [DOI] [PubMed] [Google Scholar]
- 15.Rayman MP. Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 16.Tang JY, Cao L, Jia G, Liu GM, Chen XL, Tian G, et al. The protective effect of selenium from heat stress-induced porcine small intestinal epithelial cell line (IPEC-J2) injury is associated with regulation expression of selenoproteins. Brit J Nutr. 2018;122:1081–1090. doi: 10.1017/S0007114519001910. [DOI] [PubMed] [Google Scholar]
- 17.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R. Characterization of mammalian sele-noproteomes. Science. 2003;300(5624):1439–43. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 18.Reeves MA, Hoffmann PR. The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci. 2009;66(15):2457–2478. doi: 10.1007/s00018-009-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnurr K, Belkner J, Ursini F, Schewe T, Kuhn H. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase controls the activity of the 15-lipoxygenase with complex substrates and preserves the specificity of the oxygenation products. J Biol Chem. 1996;271(9):4653–4658. doi: 10.1074/jbc.271.9.4653. [DOI] [PubMed] [Google Scholar]
- 20.Jurynec MJ, Xia R, Mackrill JJ, Gunther D, Crawford T, Flanigan KM, et al. Selenoprotein N is required for ryanodine receptor calcium release channel activity in human and zebrafish muscle. P Natl Acad Sci USA. 2008;105(34):12485–12490. doi: 10.1073/pnas.0806015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fomenko DE, Gladyshev VN. CxxS: Fold-independent redox motif revealed by genome-wide searches for thiol/disulfide oxidoreductase function. Protein Sci. 2010;11(10):2285–2296. doi: 10.1110/ps.0218302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emer K, Greene CM, Carroll TP. Selenoprotein S/SEPS1 modifies endoplasmic reticulum stress in z variant α1-antitrypsin deficiency. J Biol Chem. 2009;284(25):16891–16897. doi: 10.1074/jbc.M109.006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Tang JY, He Y, Jia G, Liu GM, Tian G, et al. Selenogenome and AMPK signal insight into the protective effect of dietary selenium on chronic heat stress-induced hepatic metabolic disorder in growing pigs. J Anim Sci Biotechno. 2021;12(1):68. doi: 10.1186/s40104-021-00590-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang LQ, Jing JZ, Yan H, Tang JY, Jia G, Liu GM, et al. Selenium pretreatment alleviated LPS-induced immunological stress via up-regulation of several selenoprotein encoding genes in murine RAW264.7 cells. Biol Trace Elem Res. 2018;186:505–13. doi: 10.1007/s12011-018-1333-y. [DOI] [PubMed] [Google Scholar]
- 25.Sunde RA, Raines AM. Selenium regulation of the selenoprotein and nonselenoprotein transcriptomes in rodents. Adv Nutr. 2011;2(2):138–150. doi: 10.3945/an.110.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang JY, He Z, Liu TG, Jia G, Liu GM, Chen XL, et al. Effect of supplementing hydroxy selenomethionine on meat quality of yellow feather broiler. Poultry Sci. 2021;100(10):101389. doi: 10.1016/j.psj.2021.101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Yin SG, Tang JY, Liu YG, Jia G, Liu GM, et al. Hydroxy selenomethionine improves meat quality through optimal skeletal metabolism and functions of selenoproteins of pigs under chronic heat stress. Antioxidants. 2021;10(10):1558. doi: 10.3390/antiox10101558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xing HJ, Zheng SF, Zhang ZW, Zhu FT, Xue H, Xu SW. Pharmacokinetics of selenium in healthy piglets after different routes of administration: application of pharmacokinetic data to the risk assessment of selenium. Biol Trace Elem Res. 2019;191:403–11. doi: 10.1007/s12011-019-1644-7. [DOI] [PubMed] [Google Scholar]
- 29.Yuan SB, Chen DW, Zhang KY, Yu B. Effects of oxidative stress on growth performance, nutrient digestibilities and activities of antioxidative enzymes of weanling pigs. Asian Australas J Anim Sci. 2008;20(10):1600–1605. doi: 10.5713/ajas.2007.1600. [DOI] [Google Scholar]
- 30.Callan JJ, Garry BP, Doherty JVO. The effect of expander processing and screen size on nutrient digestibility, growth performance, selected faecal microbial populations and faecal volatile fatty acid concentrations in grower-finisher pigs (Chinese) Anim Feed Sci Tech. 2007;134:223–234. doi: 10.1016/j.anifeedsci.2006.09.018. [DOI] [Google Scholar]
- 31.National Health and Family Planning Commission of the PR . Method for analysis of hygienic standard of edible oils (Chinese). GB 5009.227–2016. Beijing: Standardization Administration; 2016. [Google Scholar]
- 32.National Health and Family Planning Commission of the PRC . Method for analysis of hygienic standard of pastry (Chinese). GB 5009.229–2016. Beijing: Standardization Administration; 2016. [Google Scholar]
- 33.General Administration of Quality Supervision, Inspection and Quarantine of the PRC . Animal and vegetable fats and oils-determination of iodine value (Chinese). GB/T 5532–2008. Standardization Administration: Beijing; 2008. [Google Scholar]
- 34.General Administration of Quality Supervision, Inspection and Quarantine of the PRC . Animal and vegetable fats and oils-determination of saponification value (Chinese). GB/T 5534–2008. Standardization Administration: Beijing; 2008. [Google Scholar]
- 35.General Administration of Quality Supervision, Inspection and Quarantine of the PRC . Determination of selenium in feeds (Chinese). GB/T 13883–2008. Beijing: Standardization Administration; 2008. [Google Scholar]
- 36.National Health commission of the PRC . National food safety standard determination of selenium in foods (Chinese). GB 5009.93–201. Beijing: Standardization Administratio; 2010. [Google Scholar]
- 37.Zhao H, Li K, Tang JY, Zhou JC, Wang KN, Xia XJ, et al. Expression of selenoprotein genes is affected by obesity of pigs fed a high-fat diet. J Nutr. 2015;145:1394–1401. doi: 10.3945/jn.115.211318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez MJ, Gray JI, Schemmel RA, Dugan L, Welsch CW. Lipid peroxidation products are elevated in fish oil diets even in the presence of added antioxidants. J Nutr. 1992;122(11):2190–2195. doi: 10.1093/jn/122.11.2190. [DOI] [PubMed] [Google Scholar]
- 39.Ali MA, Islam MA, Othman NH, Noor AM, Hossen J, Ibrahim M. Effect of heating on compositional characteristics and oxidative stability of crude and refined rice bran oil. J Oleo Sci. 2019;68(11):1085–1097. doi: 10.5650/jos.ess19140. [DOI] [PubMed] [Google Scholar]
- 40.Wang YX, Wang WC, Yang HX, Shao D, Zhao XF, Zhang GD. Intraperitoneal injection of 4-hydroxynonenal (4-HNE), a lipid peroxidation product, exacerbates colonic inflammation through activation of Toll-like receptor 4 signaling. Free Radical Bio Med. 2019;131:237–242. doi: 10.1016/j.freeradbiomed.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 41.Ibáñez-Escriche N, Reixach J, Lleonart N, Lleonart N, Noguera JL. Genetic evaluation combining purebred and crossbred data in a pig breeding scheme. J Anim Sci. 2011;89(12):3881–3889. doi: 10.2527/jas.2011-3959. [DOI] [PubMed] [Google Scholar]
- 42.Ruusunen R, Puolanne E. Histochemical properties of fibre types in muscles of wild and domestic pigs and the effect of growth rate on muscle fibre properties. Meat Sci. 2004;67(3):533–539. doi: 10.1016/j.meatsci.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17(9):422–427. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radical Bio Med. 1999;26:463–471. doi: 10.1016/S0891-5849(98)00216-0. [DOI] [PubMed] [Google Scholar]
- 45.Wang P, Deng JW, Dong J, Liu JH, Bigio EH, Mesulam M, et al. Tdp-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response. Plos Genet. 2019;15(5):1–32. doi: 10.1371/journal.pgen.1007947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naresh NU, Haynes CM. Signaling and regulation of the mitochondrial unfolded protein response. CSH Perspect Biol. 2019;11(6):a033944. doi: 10.1101/cshperspect.a033944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haynes CM, Christopher JF, Lin YF. Evaluating and responding to mitochondrial dysfunction: the mitochondrial unfolded-protein response and beyond. Trends Cell Biol. 2013;23(7):311–318. doi: 10.1016/j.tcb.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rath E, Haller D, Moschetta A. Mitochondrial function–gatekeeper of intestinal epithelial cell homeostasis. Nat Rev Gastro Hepat. 2018;15(8):497–516. doi: 10.1038/s41575-018-0021-x. [DOI] [PubMed] [Google Scholar]
- 49.Aldridge JE, Horibe T, Hoogenraad NJ. Discovery of genes activated by the mitochondrial unfolded protein response (mtUPR) and cognate promoter elements. PLoS One. 2007;2(9):e874. doi: 10.1371/journal.pone.0000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Q, Wang JH, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21(17):4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiwon H, Ling Q. Quality control in the endoplasmic reticulum: crosstalk between ERAD and UPR pathways. Trends Biochem Sci. 2018;43(8):593–605. doi: 10.1016/j.tibs.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BIP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3(1):99–111. doi: 10.1016/S1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 53.Sandri M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell B. 2013;45(10):2121–2129. doi: 10.1016/j.biocel.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maria G, Pawel AO. Targeting protein-protein Interactions in the ubiquitin-proteasome pathway. Adv Protein Chem Str. 2018;110:123–165. doi: 10.1016/bs.apcsb.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 55.Wang XM, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology. 2007;21(5):362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 56.Böhm R, Imseng S, Jakob RP. The dynamic mechanism of 4E-BP1 recognition and phosphorylation by mTORC1. Mol Cell. 2021;81(11):2403–2416. doi: 10.1016/j.molcel.2021.03.031. [DOI] [PubMed] [Google Scholar]
- 57.Xie XD, Hu HL, Tong XY, Li L, Liu XY, Chen M, et al. The mTOR-S6K pathway links growth signalling to DNA damage response by targeting RNF168. Nat Cell Biol. 2018;20(3):320–331. doi: 10.1038/s41556-017-0033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Semenkovich CF. Regulation of fatty acid synthase (FAS) Prog Lipid Res. 1997;36(1):43–53. doi: 10.1016/S0163-7827(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 59.Zhang YQ, Yin LY, Hillgartner FB. SREBP-1 integrates the actions of thyroid hormone, insulin, camp, and medium-chain fatty acids on accalpha transcription in hepatocytes. J Lipid Res. 2003;44(2):356–368. doi: 10.1194/jlr.M200283-JLR200. [DOI] [PubMed] [Google Scholar]
- 60.Gosmain Y, Dif N, Berbe V, Loizon E, Rieusset J, Vidal H, et al. Regulation of SREBP-1 expression and transcriptional action on HKII and FAS genes during fasting and refeeding in rat tissues. J Lipid Res. 2005;46(4):697–705. doi: 10.1194/jlr.M400261-JLR200. [DOI] [PubMed] [Google Scholar]
- 61.Kershaw EE, Hamm JK, Verhagen LAW, Peroni O, Katic M, Flier JS. Adipose triglyceride lipase: Function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55(1):148–157. doi: 10.2337/diabetes.55.01.06.db05-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang JY, Wang LQ, Jia G, Liu GM, Chen XL, Tian G, et al. The hydroxy-analogue of selenomethionine alleviated lipopolysaccharide-induced inflammatory responses is associated with recover expression of several selenoprotein encoding genes in the spleens of Kunming mice. RSC Adv. 2019;9(69):40462–40470. doi: 10.1039/C9RA07260H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han SJ, Lee BC, Yim SH, Gladyshev VN, Lee SR. Characterization of mammalian selenoprotein O: a redox-active mitochondrial protein. PLoS One. 2014;9(4):e95518. doi: 10.1371/journal.pone.0095518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaya A, Lee BC, Gladyshev VN. Regulation of protein function by reversible methionine oxidation and the role of selenoprotein Msrb1. Antioxid Redox Sign. 2015;23(10):814–822. doi: 10.1089/ars.2015.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fomenko DE, Novoselov SV, Natarajan SK, Lee BC, Koc A, Carlson BA, et al. Msrb1 (methionine-r-sulfoxide reductase 1) knock-out mice: roles of msrb1 in redox regulation and identification of a novel selenoprotein form. J Biol Chem. 2009;284(9):5986–5993. doi: 10.1074/jbc.M805770200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mujahid A, Akiba Y, Toyomizu M. Olive oil-supplemented diet alleviates acute heat stress-induced mitochondrial ROS production in chicken skeletal muscle. Am J Physiol-Reg I. 2009;297(3):R690–R698. doi: 10.1152/ajpregu.90974.2008. [DOI] [PubMed] [Google Scholar]
- 67.Santesmasses D, Mariotti M, Gladyshev VN. Tolerance to selenoprotein loss differs between human and mouse. Mol Biol Evol. 2020;37(2):341–354. doi: 10.1093/molbev/msz218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pitts MW, Hoffmann PR. Endoplasmic reticulum-resident selenoproteins as regulators of calcium signaling and homeostasis. Cell Calcium. 2018;70:76–86. doi: 10.1016/j.ceca.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ren BY, Liu M, Ni JZ, Tian J. Role of selenoprotein F in protein folding and secretion: Potential involvement in human disease. Nutrients. 2018;10(11):1619. doi: 10.3390/nu10111619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deniziak M, Thisse C, Rederstorff M, Hindelang C, Thisse B, Lescure A. Loss of selenoprotein N function causes disruption of muscle architecture in the zebrafish embryo. Exp Cell Res. 2007;313(1):156–167. doi: 10.1016/j.yexcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 71.Zeng JH, Du SQ, Zhou J, Huang KX. Role of SelS in lipopolysaccharide-induced inflammatory response in hepatoma HepG2 cells. Arch Biochem Biophys. 2008;478(1):1–6. doi: 10.1016/j.abb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 72.Zhou J, Du S, Jia Y, Huang KX. SelK is a novel ER stress-regulated protein and protects HepG2 cells from ER stress agent-induced apoptosis. Arch Biochem Biophys. 2010;502(2):137–143. doi: 10.1016/j.abb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Jia SZ, Xu XW, Zhang ZH, Chen C, Chen YB, Huang SL, et al. Selenoprotein K deficiency-induced apoptosis: A role for calpain and the ERS pathway. Redox Biol. 2021;47:102154. doi: 10.1016/j.redox.2021.102154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang J, Bao D, Lei CT, Tang H, Zhang CY, Su H, et al. Selenoprotein T protects against cisplatin-induced acute kidney injury through suppression of oxidative stress and apoptosis. Faseb J. 2020;34(9):11983–96. doi: 10.1096/fj.202000180RR. [DOI] [PubMed] [Google Scholar]
- 75.Gong T, Hashimoto AC, Sasuclark AR, Khadka VS, Gurary A, Pitts MW. Selenoprotein M promotes hypothalamic leptin signaling and thioredoxin antioxidant activity. Antioxid Redox Sign. 2019;35(10):775–787. doi: 10.1089/ars.2018.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Composition and nutrient levels of the basal diet. Table S2. The oxidation Characteristics of the diets. Table S3. Primers used for the Q-PCR. Table S4. Primary antibodies for the Western blot analyses. Table S5. The correlation analysis between the 12 key selenogenes and other measures.
Additional file 2: Fig. S1. Diet Se concentration.
Additional file 3: Fig. S2. The mRNA expression of the 22 selenogenes.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.