Abstract
Background
Isolated small airway abnormalities may be demonstrable at rest in patients with normal spirometry; however, the relationship of these abnormalities to exertional symptoms remains uncertain. This study uses an augmented cardiopulmonary exercise test (CPET) to include evaluation of small airway function during and following exercise to unmask abnormalities not evident with standard testing in individuals with dyspnoea and normal spirometry.
Methods
Three groups of subjects were studied: 1) World Trade Center (WTC) dust exposure (n=20); 2) Clinical Referral (n=15); and Control (n=13). Baseline evaluation included respiratory oscillometry. Airway function during an incremental workload CPET was assessed by: 1) tidal flow versus volume curves during exercise to assess for dynamic hyperinflation and expiratory flow limitation; and 2) post-exercise spirometry and oscillometry to evaluate for airway hyperreactivity.
Results
All subjects demonstrated normal baseline forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC). Dyspnoea was reproduced during CPET in WTC and Clinical Referral groups versus Control without abnormality in respiratory pattern and minute ventilation. Tidal flow–volume curves uncovered expiratory flow limitation and/or dynamic hyperinflation with increased prevalence in WTC and Clinical Referral versus Control (55%, 87% versus 15%; p<0.001). Post-exercise oscillometry uncovered small airway hyperreactivity with increased prevalence in WTC and Clinical Referral versus Control (40%, 47% versus 0%, p<0.05).
Conclusions
We uncovered mechanisms for exertional dyspnoea in subject with normal spirometry that was attributable to either small airway dysfunction during exercise and/or small airway hyperreactivity following exercise. The similarity of findings in WTC environmentally exposed and clinically referred cohorts suggests broad relevance for these evaluations.
Short abstract
Augmentation of cardiopulmonary exercise test (CPET) to include assessment of airway function unmasked treatable mechanisms for dyspnoea related to small airway dysfunction during and/or following exercise in symptomatic patients despite normal spirometry https://bit.ly/3KST6Jw
Introduction
Exertional dyspnoea is frequently encountered and may remain unexplained by standard clinical evaluation. Spirometry may fail to show abnormalities when disease is localised to the small airways [1, 2]. This “quiet zone” of the lung minimally impacts airflow because it has a large aggregate cross-sectional area, and non-spirometric techniques have been required to document evidence for small airway injury in symptomatic patients [2]. Data obtained with respiratory oscillometry link small airway dysfunction to respiratory symptoms, presence of local and systemic inflammation, imaging abnormalities, inhaled toxin exposure, histological abnormalities and response to therapy [3–10].
Small airway dysfunction may be identified at rest, but the relationship to symptoms that are evident predominately on exertion remains uncertain. This study is based on the overarching theme that examination of small airway function, particularly during exercise, may provide mechanism(s) for exertional dyspnoea in subjects despite normal airflow on spirometry. This line of investigation is relevant to assessment of dyspnoea in numerous disease states, including pre-COPD, asthma and inhaled toxin-induced airway injury prior to development of resting spirometric abnormalities.
Markers of small airway dysfunction during exercise can be obtained by augmenting standard cardiopulmonary exercise test (CPET) protocols to include tidal flow versus volume curves during exercise to identify expiratory flow limitation and/or dynamic hyperinflation [11–15]. Application of this technique has been recommended in recent CPET guidelines and has been applied to patients with established lung disease, but equipment may not be available in all centres [16–18]. Additionally, exercise-induced airway hyperreactivity has been described using post-exercise spirometry [19], but given the limitations of spirometry, the role for specific assessment of small airway function using post-exercise oscillometry has yet to be defined.
We hypothesise that: 1) expanding CPET protocols to include evaluation of small airway function during and following exercise may unmask abnormalities not evident with standard exercise testing providing mechanisms for unexplained dyspnoea; and 2) presence of small airway dysfunction at rest via spirometry and/or oscillometry is predictive of exercise-induced airway dysfunction. Two cohorts of symptomatic patients with normal spirometry were included. First, subjects were enrolled from a funded prospective cohort of symptomatic individuals at risk for airway disease solely due to exposure to dust and debris from collapse of the World Trade Center (WTC) towers. Second, a clinical cohort was retrospectively identified with other potential risks of exertional dyspnoea. These two cohorts were combined with the sponsor's approval (Centers for Disease Control and Prevention (CDC)/National Institute of Occupation Safety and Health (NIOSH)) to highlight the robustness of this approach in a broad range of symptomatic individuals.
Methods
Subjects
The retrospective clinical referral cohort included individuals with exertional dyspnoea unexplained by underlying cardiorespiratory disease and normal airflow on spirometry (Clinical Referral, n=15). The prospective funded study recruited symptomatic community members enrolled in the WTC Environmental Health Center (WTC Dust, n=20). Inclusion criteria included new onset and persistent lower respiratory symptoms following WTC dust exposure that remained uncontrolled (Asthma Control Test score <20), despite treatment with inhaled corticosteroids (ICS) and long-acting β-agonist (LABA), normal spirometry airflow and <5 pack-years smoking history. Asymptomatic control subjects (n=13) were recruited from both clinical referrals and WTC dust-exposed populations based on absence of respiratory symptoms and medications, <5 pack-years smoking history and normal spirometry airflow. For all cohorts, normal airflow was defined as values for forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and FEV1/FVC that were above the lower limits of normal. Individuals with prior lung disease, unstable cardiac disease, cardiomyopathy or history of heart failure were excluded. This study was approved by the Institutional Review Board of NYU School of Medicine and Bellevue Hospital.
Study design
All subjects underwent resting evaluation using spirometry, plethysmography, diffusing capacity of the lung for carbon monoxide (DLCO) and oscillometry. CPET with assessment of airway function during and following exercise was performed within 2 weeks. Respiratory medications were not withheld prior to the CPET to evaluate exertional symptoms under the chronic medical regime.
Resting lung function
Spirometry, lung volumes and diffusion were performed in accordance with published standards and normative values (Vmax Encore; SensorMedics, Yorba Linda, CA, USA) [20–25]. Subtle indicators that could reflect small airway dysfunction on spirometry included maximal mid-expiratory airflow between 25% and 75% of the vital capacity (MMEF) and tidal expiratory flow limitation identified visually as superimposition of the exhaled tidal airflow on the maximal forced expiratory airflow for at least 40% of the tidal volume. Data for lung volumes and DLCO were obtainable in 45 out of 48 subjects.
Oscillometry was performed in accordance with published standards (Jaeger Impulse Oscillation System; Jaeger USA, Yorba Linda, CA, USA) [26]. Measurements were obtained during tidal breathing with a nose clip and cheek support. Parameters included: 1) resistance at oscillating frequencies of 5 Hz (R5) and 20 Hz (R20); 2) frequency dependence of resistance calculated as R5 minus R20 (R5–20); and 3) reactance area (AX) calculated as the area above the reactance curve from 5 Hz to the resonant frequency. Trials with glottis closure and mouth leak were excluded. Data are presented as raw values and compared to normative data [27].
Cardiopulmonary exercise test
CPET was performed on a treadmill with incremental workload (Bruce protocol). Electrocardiogram and breath-by-breath expired gas analysis (Viasys Vmax, Yorba Linda, USA) were recorded during a 2-min pre-exercise period and throughout exercise. Peak oxygen uptake (V′O2) was determined at maximal exertion and compared with normative values [28]. The exercise protocol included: 1) tidal flow versus volume curves at each stage of exercise to evaluate for development of expiratory flow limitation (>40% of tidal volume) and/or dynamic hyperinflation (reduction in inspiratory capacity >0.15 L) [16, 17]; and 2) post-exercise airway hyperreactivity assessed by serial spirometry and oscillometry obtained at 3-min intervals for 15 min. Airway hyperreactivity was defined on spirometry as a ≥10% fall in FEV1 [19] and on oscillometry by the upper limit of normal for change in post-exercise R5 derived from the Control group.
Statistical analysis
Data were summarised as mean±sd. Differences between groups were analysed using ANOVA. Post hoc pair-wise testing utilised Tukey's honestly significant difference test to evaluate differences between the Clinical Referral or WTC as compared with Control. Chi-squared and Fisher's exact test were used to assess for differences in the prevalence of resting and exercise small airway abnormalities between both the Clinical Referral and WTC groups as compared with Control. Data were analysed using SPSS v25.
Results
Table 1 illustrates the characteristics of the study population. Control subjects tended to be younger than study groups. Height, weight and body mass index (BMI) were similar across groups. By definition, all controls were never-smokers and were asymptomatic despite WTC dust exposure in some individuals. Numerous risk factors for exertional dyspnoea were evident in the WTC Dust and Clinical Referral groups with variable prevalence of cigarette smoking, environmental/workplace exposure and underlying comorbidities. Approximately half of the Clinical Referral cohort demonstrated persistent dyspnoea despite inhaled controller therapy. By definition, exertional dyspnoea persisted despite ICS/LABA therapy in all WTC Dust subjects.
TABLE 1.
Characteristics of the study population
| Control subjects | WTC Dust | Clinical referral | |
| Subjects n | 13 | 20 | 15 |
| Age years | 46±18 | 58±11* | 58±13 |
| Height m | 1.7±0.1 | 1.6±0.1 | 1.7±0.1 |
| Weight kg | 79.4±20.4 | 82.6±16.3 | 80.7±14.5 |
| BMI kg·m−2 | 27±6 | 32±7 | 28±4 |
| Inhalational exposure | |||
| Cigarette smoking (>5 pack-years) | 0 (0) | 0 (20) | 3 (15) |
| Environmental/work | 7 (13) | 20 (20) | 5 (15) |
| Comorbid conditions | |||
| Hypertension and/or atherosclerosis | 4 (13) | 8 (20) | 8 (15) |
| Musculoskeletal | 0 (13) | 0 (20) | 5 (15) |
| Oncologic | 3 (13) | 2 (20) | 3 (15) |
| Medications | |||
| ICS/LABA and/or LAMA | 0 (13) | 20 (20) | 7 (15) |
| SABA | 0 (16) | 20 (20) | 8 (15) |
Data are reported as mean±sd or n (%) unless otherwise indicated. WTC: World Trade Center; BMI: body mass index; ICS: inhaled corticosteroid; LABA: long-acting β-agonist; LAMA: long-acting muscarinic antagonist; SABA: short-acting β-agonist. *: p<0.05 versus control.
Resting lung function
Table 2 illustrates the baseline lung function data. The Control group demonstrated normal mean values for spirometry metrics. The WTC Dust cohort data were similar to control; while the Clinical Referral group had lower FEV1 and FEV1/FVC, by definition, data were within the normal range in all subjects. Despite normal FEV1/FVC in WTC Dust and Clinical Referral cohorts, small airway dysfunction was suggested by a trend towards higher prevalence of expiratory flow limitation during tidal breathing as compared with Controls (table 3; Control 23%, WTC Dust 45%, Clinical Referral 47%). Subjects with expiratory flow limitation demonstrated similar age to those without flow limitation. Reduction in MMEF was observed in only three symptomatic subjects. Mean values for lung volumes and DLCO were within normal limits without differences between groups; abnormal DLCO was noted in five symptomatic subjects. Further analyses related the alveolar volume (VA) measured during the DLCO test to the total lung capacity (TLC) measured in the plethysmograph; values for VA/TLC <85% suggestive of non-uniform of gas distribution were noted with similar frequency across groups.
TABLE 2.
Baseline lung function data in the study population
| Control subjects | WTC Dust | Clinical referral | |
| Subjects n | 13 | 20 | 15 |
| Spirometry | |||
| FEV1 % pred | 102±17 | 101±14 | 87±12* |
| FVC % pred | 104±16 | 101±15 | 94±13 |
| FEV1/VC | 79±6 | 79±5 | 72±5* |
| MMEF % pred | 96±28 | 107±32 | 71±19 |
| Lung volumes | |||
| TLC % pred | 97±17 | 91±15 | 97±13 |
| ERV % pred | 118±42 | 94±45 | 79±43 |
| IC % pred | 96±23 | 93±23 | 96±16 |
| Diffusion capacity | |||
| DLCO % pred | 93±17 | 96±20 | 81±13 |
| KCO mL·min−1·mmHg−1·L−1 | 4.47±0.70 | 4.98±0.94 | 3.98±0.69 |
| Oscillometry | |||
| R5 cmH2O·L−1·s | 3.69±1.16 | 4.87±1.77 | 4.23±1.26 |
| R20 cmH2O·L−1·s | 3.11±1.00 | 3.54±1.10 | 3.24±1.00 |
| R5-20 cmH2O·L−1·s | 0.59±0.59 | 1.33±1.01* | 1.00±0.45 |
| AX cmH2O·L−1 | 5.09±5.07 | 14.11±10.88* | 8.54±4.54 |
Data are reported as mean±sd unless indicated otherwise. WTC: World Trade Center; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FEV1/VC: ratio of forced expiratory volume in 1 s to vital capacity; MMEF: maximal mid-expiratory airflow between 25% and 75% of the vital capacity; TLC: total lung capacity; ERV: expiratory reserve volume; IC: inspiratory capacity; DLCO: diffusion capacity of the lungs for carbon monoxide; KCO: transfer coefficient of the lung for carbon monoxide; R5: resistance of oscillating frequency at 5 Hz; R20: resistance of oscillating frequency at 20 Hz; R5-20: frequency dependence of resistance; AX: reactance area. *: p<0.05 versus control.
TABLE 3.
Prevalence of resting and exercise airway abnormalities in the study groups
| Control subjects | WTC Dust | Clinical referral | |
| Subjects n | 13 | 20 | 15 |
| Baseline abnormalities | |||
| Expiratory flow limitation | 3/13 (23) | 9/20 (45) | 7/15 (47) |
| Non-uniform gas distribution# | 5/12 (42) | 9/18 (50) | 9/15 (60) |
| Abnormal oscillometry | 2/13 (15) | 15/20 (75)* | 12/15 (80)* |
| Exercise abnormalities | |||
| Expiratory flow limitation | 2/13 (15) | 10/20 (50)* | 12/15 (80)* |
| Dynamic hyperinflation | 1/13 (8) | 4/20 (20) | 2/15 (13) |
| Any abnormality | 2/13 (15) | 11/20 (55)* | 13/15 (87)* |
| Post-exercise abnormalities | |||
| Reduced FEV1 | 1/13 (8) | 3/20 (15) | 3/15 (20) |
| Increased R5 | 0/13 (0) | 8/20 (40)* | 7/15 (47)* |
| Any abnormality | 1/13 (8) | 11/20 (55)* | 10/15 (67)* |
| Any exercise or post-exercise abnormality | 3/13 (23) | 16/20 (80)* | 14/15 (93)* |
Data are presented as n/N (%) unless otherwise indicated. WTC: World Trade Center; FEV1: forced expiratory volume in 1 s; R5: resistance of oscillating frequency at 5 Hz. #: data are shown for the 45 out of 48 individuals that were able to complete the plethysmography and diffusion capacity tests. *: p<0.05 versus control.
Oscillometry data are shown in table 2. The Control group demonstrated normal mean values for all parameters; the WTC Dust and Clinical Referral groups demonstrated higher values that reached statistical significance for R5-20 and AX in the WTC Dust group. Increased prevalence of abnormal R5 and/or AX was noted in the WTC Dust and Clinical Referral groups versus Control (table 3: Control 15%, WTC Dust 75%, Clinical Referral 80%; p<0.001); concomitant elevation of R5-20, suggested small airway dysfunction. The observed abnormalities are not attributable to body weight since the normative data accounts for excess body weight.
Cardiopulmonary exercise test
Table 4 illustrates CPET data. The Borg dyspnoea score in Control increased from 0 at rest to 3.1±2.3 at peak exertion. The WTC Dust group had significantly increased dyspnoea at baseline compared with Control (1.4±1.5, p<0.05). Augmentation of dyspnoea was noted in the WTC Dust group during exercise; while the peak Borg score did not differ from Control, subjects terminated exercise at lower peak V′O2 (Control 100±28% predicted versus WTC Dust 82±12% predicted, p<0.05) and lower peak respiratory quotient (RQ) (Control 1.26±0.13 versus WTC Dust 1.16±0.10, p<0.05). The Clinical Referral subjects had minimal dyspnoea at baseline; these subjects exercised to workloads similar to Control, as assessed by peak V′O2, but with increased dyspnoea (5.8±2.5; p<0.005). Mean values for standard cardiac and ventilatory parameters at peak exercise (heart rate, O2 pulse, respiratory rate, tidal volume (VT), VT/inspiratory capacity (IC), minute ventilation (V′E) and V′E/maximum voluntary ventilation) were within the normal range in all groups, excluding cardiac or ventilatory limitation by traditional metrics despite reproduction of symptoms. Lower values for peak cardiac and ventilatory parameters were noted in the WTC Dust cohort compared with Control in accord with termination of exercise due to symptom onset at lower peak V′O2. Electrocardiographic abnormalities were not noted in any subject. Additional gas exchange parameters of oxygen saturation, V′E/carbon dioxide production (V′CO2), V′E/V′O2 and end-tidal carbon dioxide tension (PETCO2), were normal in all subjects (data not shown).
TABLE 4.
Cardiopulmonary exercise test data for the study population
| Control subjects | WTC Dust | Clinical referral | |
| Subjects n | 13 | 20 | 15 |
| Baseline Borg dyspnoea | 0.0±0.0 | 1.4±1.5* | 0.8±1.5 |
| Peak Borg dyspnoea | 3.1±2.3 | 4.2±2.3 | 5.8±2.5* |
| P eak V′O2 % pred | 100±28 | 82±12* | 91±14 |
| Peak HR % pred | 93±10 | 81±11* | 90±12 |
| Peak RR breaths·min−1 | 38±12 | 36±6 | 41±14 |
| Peak VT L | 2.21±0.70 | 1.35±0.62* | 1.88±0.46 |
| Peak VT / VC % | 50±8 | 42±7 | 52±13 |
| Peak V′E L·min−1 | 88±26 | 52±22* | 80±23 |
| Peak V′E/MVV % | 74±29 | 54±16* | 77±19 |
| Peak RQ | 1.26±0.13 | 1.16±0.10* | 1.15±0.11* |
| V′E/V′CO2 (at anaerobic threshold) | 28.5±3.2 | 29.8±3.7 | 31.2±2.2 |
| O2 pulse % pred | 114±36 | 99±15 | 110±23 |
| Anaerobic threshold (% of pred V′O2 max) | 57±11 | 56±7 | 57±9 |
Data are reported as mean±sd. V′O2: peak oxygen uptake; HR: heart rate; RR: respiratory rate; VT : tidal volume; V′E: minute ventilation; MVV: maximum voluntary ventilation. *: p<0.05 versus control.
Airway function during and following exercise
Tidal flow–volume curves during exercise uncovered expiratory flow limitation and/or dynamic hyperinflation in 15% of Control subjects versus 55% WTC Dust and 87% of Clinical Referral subjects (table 3; p<0.001), indicating airway dysfunction not identifiable by the aforementioned standard respiratory pattern and gas exchange parameters.
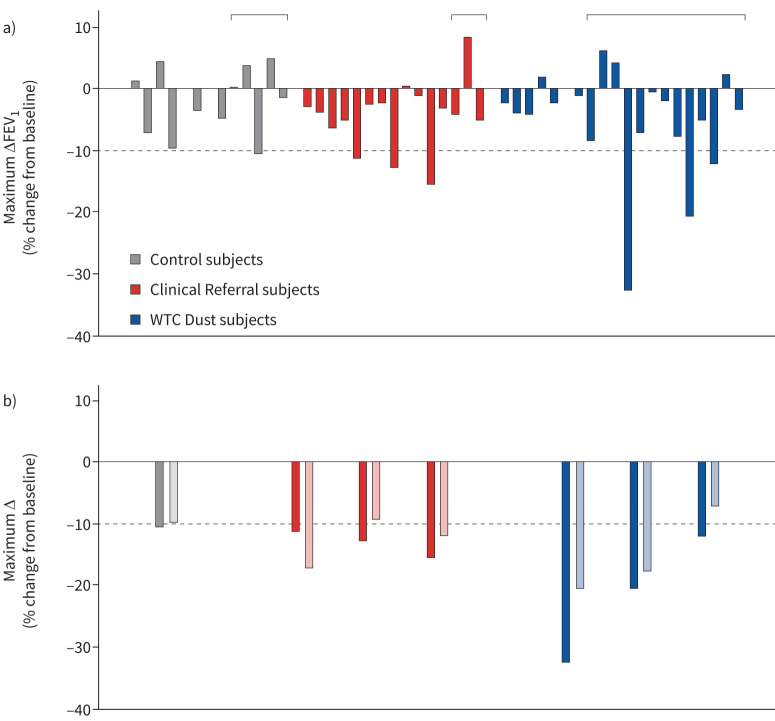
Exercise-induced airway hyperreactivity was assessed using post-exercise spirometry. Figure 1 (top panel) illustrates the maximal change in FEV1 following exercise. Airway hyperreactivity was demonstrated in WTC Dust and Clinical Referral cohorts slightly more frequently than in Controls (table 3; 15% and 20% versus 8%). The bottom panel demonstrates that reduction in FEV1 in these individuals occurred with parallel reduction in FVC, consistent with small airway closure [29].
FIGURE 1.
a) Maximum % change in forced expiratory volume in 1 s (FEV1) in each subject following exercise. The dashed line represents the lower limit of normal. b) Maximum % change in FEV1 (solid bars) and forced vital capacity (FVC) (lightly shaded bars) following exercise in the seven subjects that demonstrated ≥10% decline in FEV1 from baseline. Subjects within each group are arranged by increasing body mass index (BMI). Braces depict subjects with BMI ≥30 kg·m−2. WTC: World Trade Center.
Exercise-induced airway hyperreactivity was also assessed using post-exercise oscillometry. Threshold oscillometry values indicative of exercise-induced airway hyperreactivity are unknown, and a potential influence of obesity on oscillometry outcomes is evident from methacholine challenge data [29–35]. Accordingly, figure 2 plots the maximal increase in R5 post exercise as a function of BMI. The shaded area depicts the 95% confidence interval for observations in Control confirming presence of increased reactivity with increasing BMI (R2=054; p<0.005). The coloured dots illustrate data for individual subjects within the WTC Dust and Clinical Referral groups; an increased prevalence of airway hyperreactivity was noted in WTC Dust and Clinical Referral versus Control (table 3; Control 0%, WTC Dust 40%, Clinical Referral 47%; p<0.05); the majority of these subjects were not obese (BMI <30 kg·m−2). When data are analysed across groups, individuals with post-exercise airway hyperreactivity demonstrated a greater increase in R5 as compared with individuals without airway hyperreactivity (1.21±0.95 versus 0.32±0.47 cmH2O·L−1·s, p<0.001). The increased R5 was associated with increased R5-20 (0.63±0.82 versus 0.31±0.32 cmH2O·L−1·s, p=0.06) and AX (7.29±11.96 versus 2.79±4.70 cmH2O·L−1, p=0.07), consistent with small airway hyperreactivity. The 15 subjects with hyperreactivity on oscillometry did not exhibit hyperreactivity on spirometry. Oscillometry abnormalities reversed to, or below, baseline in nine subjects that received inhaled β-agonist after exercise.
FIGURE 2.
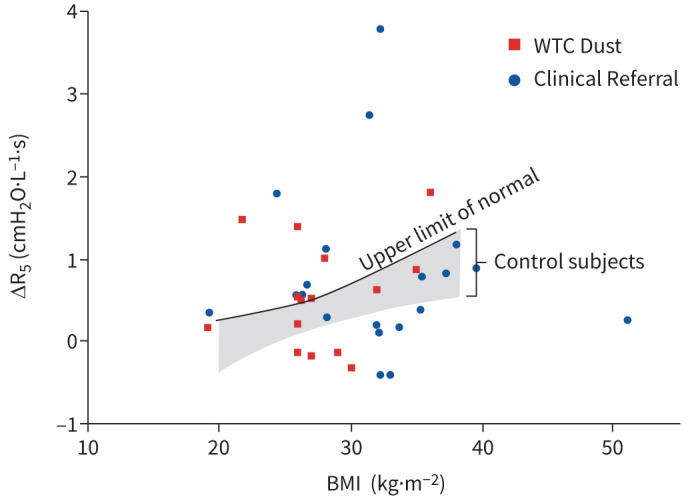
The maximum change in R5 following exercise is plotted as a function of body mass index (BMI). The shaded area depicts the predicted values and the 95% confidence interval for maximum change in R5 derived in Controls. Data points above the shaded area indicate individuals with hyperreactivity identified by R5. WTC: World Trade Center.
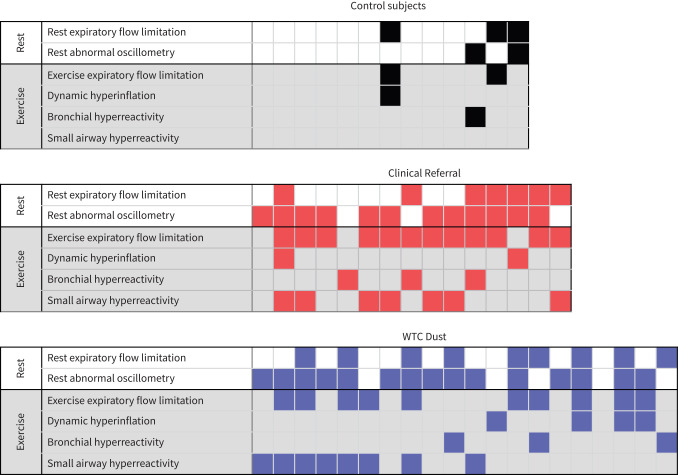
Figure 3 illustrates individual subject data summarising the small airway abnormalities unmasked during this study. One or more small airway abnormalities were identified in response to exercise in nearly all symptomatic subjects that were not evident on resting FEV1 or standard exercise functional parameters (table 3; Control 23%, WTC Dust 80%, Clinical Referral 93%; p<0.001). The two most prevalent abnormalities were presence of expiratory flow limitation during exercise and identification of small airway hyperreactivity following exercise. Table 5 shows that the presence of resting abnormalities was highly sensitive for predicting exercise-induced airway dysfunction and was maximised when resting flow limitation was combined with abnormal oscillometry (sensitivity 91%, specificity 67%, positive predicted value 86%, negative predictive value 77%).
FIGURE 3.
Small airway abnormalities are charted for each individual in each study group. Each column represents an individual subject. Shaded boxes represent specific abnormalities seen in individual subjects. WTC: World Trade Center.
TABLE 5.
Sensitivity and specificity of small airway dysfunction at rest in predicting presence of an exercise-induced airway abnormality
| Sensitivity | Specificity | Positive predicted value | Negative predicted value | |
| Expiratory flow limitation | 55 | 93 | 95 | 48 |
| Abnormal oscillometry | 73 | 67 | 83 | 53 |
| Flow limitation and/or abnormal oscillometry | 91 | 67 | 86 | 77 |
Data are presented as %.
Discussion
The present study describes the role of small airway dysfunction in producing exertional dyspnoea unexplained by standard spirometry measures. Symptomatic subjects with essentially normal lung function at rest by standard metrics were studied. Results demonstrated: 1) resting small airway dysfunction was suggested by subtle spirometric abnormalities and confirmed by abnormal oscillometry in nearly all symptomatic subjects; 2) the presence of resting small airway dysfunction was highly predictive of exercise-induced small airway abnormality either during exercise (expiratory flow limitation and/or dynamic hyperinflation) and/or following exercise (small airway hyperreactivity). These findings provide mechanisms for exertional dyspnoea that may be reversible with therapy but are not evident on standard rest and exercise testing protocols. The similarity of findings in WTC environmentally exposed and clinically referred cohorts suggest broad relevance for these observations.
Resting measurements uncovered small airway dysfunction in nearly all symptomatic subjects. Subtle indicators of small airway dysfunction were identified in some subjects on resting spirometry (low MMEF and expiratory flow limitation), but these parameters are not included in current interpretation guidelines [20]. In addition, while diffusion capacity has been suggested as an early marker of COPD, this was rarely abnormal in our study. In contrast, oscillometry was the most sensitive marker of small airway dysfunction with resting abnormalities evident in nearly all symptomatic subjects. The focus of the present study was to elucidate the mechanisms linking resting small airway dysfunction to development of exertional dyspnoea using a CPET protocol that included assessment of airway function.
All subjects underwent a CPET to evaluate the aetiology for their exertional dyspnoea, yet standard parameters failed to demonstrate any functional abnormality. Dyspnoea was reproduced in the symptomatic groups with normal or minimal impairment in exercise capacity and with normal cardiac function, excluding cardiac or musculoskeletal impairment. In addition, standard CPET ventilatory pattern and gas exchange parameters were normal for all subjects despite development of dyspnoea.
Exercise tests included specific markers of airway dysfunction. Analysis of tidal flow versus volume curves during exercise may unmask abnormalities such as expiratory flow limitation and dynamic hyperinflation as initially described in individuals with established COPD and more recently described in symptomatic individuals with normal or borderline low FEV1/FVC at rest [11–15]. In the present study, although FEV1/FVC was normal in all symptomatic subjects, most individuals demonstrated a suggestion of small airway dysfunction on spirometry at rest that manifestdc predominately as expiratory flow limitation. The increased ventilatory requirements of exercise exaggerated the expiratory flow limitation in many subjects, providing a mechanism for exertional dyspnoea.
Exercise-induced airway hyperreactivity was uncovered as an additional mechanism for exertional dyspnoea. Airway hyperreactivity was identified by post-exercise fall in FEV1. In these individuals there was a parallel fall in FVC with minimal change in FEV1/FVC indicating small airway closure [29]. In addition, exercise-induced small airway hyperreactivity was demonstrable by oscillometry in a discrete group of subjects who did not meet spirometric criteria. This disparity between spirometry and oscillometry assessments of airway function likely reflects either the known limitations of spirometry in assessing small airway function that can be uncovered by oscillometry and/or the potential to detect airway closure on forced exhalation during spirometry that may not be present during resting tidal breathing [1, 2]. This disparity also matches prior studies of methacholine challenge tests where development of symptoms was more closely linked to the onset of small airway abnormalities on oscillometry than to changes in FEV1 on spirometry [36, 37]. In both the present study and in these prior methacholine challenge data sets, the airway dysfunction and symptoms were reversible with a bronchodilator, which has therapeutic implications.
An additional finding in this study is demonstration of an association between obesity and post-exercise small airway reactivity in asymptomatic individuals as seen in the Control group. Prior studies using methacholine as a provocative agent have obtained discordant results regarding the effect of obesity on airway reactivity [29, 30, 32, 38]. Nevertheless, when reactivity is demonstrable in obese subjects, it predominately manifests by oscillometry abnormalities rather than reduced spirometric airflow [29, 30, 32–35]. The observed oscillometry pattern is consistent with small airway reactivity based on increased resistance, frequency dependence of resistance and elastance. The present study extends these observations to a group of healthy subjects evaluated using exercise to provoke airway reactivity. Potential factors that could lead to enhanced reactivity in the symptomatic obese subject include presence of a reduced operating lung volume with reduced resting airway dimension, presence of central vascular congestion, impaired bronchodilatory response to deep inspiration and/or intrinsic alteration of airways [32, 34, 38–43].
The small airway dysfunction observed in symptomatic patients in this study may be attributable to a variety of factors. Subjects had variable likelihood for inhalational lung injury with WTC dust exposure present in over half of the symptomatic subjects coupled with lower prevalence of cigarette smoking and workplace exposures. Undiagnosed airway disease predominantly localised to the small airways and not evident on spirometry could be present as described in pre-COPD and asthma [27]. Obesity was highly prevalent in the symptomatic cohorts, which could have contributed to both resting and exercise-induced functional abnormalities by any of the mechanisms noted above. The precise mechanism(s) are likely to vary across patients and dictate the optimal therapeutic approach.
There are several factors to be considered when interpreting the data obtained in this study. First, although comorbid cardiac conditions were present, they were unlikely causes for exertional dyspnoea since the cardiac response to exercise was within normal limits. Comorbid musculoskeletal conditions were also present but did not limit exercise in any individual. Second, chronic inhaler therapy was empirically prescribed in several subjects and may have precluded finding abnormalities in individual subjects. Last, an incremental exercise protocol was used to assess for exercise-induced airway hyperreactivity rather than the recommended steady-state protocol at high workload. The advantage of this approach is that it allowed inclusion of additional cardiac and metabolic end-points while completing the entire evaluation in a single test. Nevertheless, the prevalence of airway hyperreactivity may have been underestimated by this protocol.
Summary
This study utilised a novel approach to assess exertional symptoms by evaluating small airway function at rest and during an augmented exercise protocol to replicate patient symptoms. One or more manifestations of small airway dysfunction were evident in nearly all symptomatic patients. Most subjects demonstrated markers of small airway dysfunction at rest that manifested as expiratory flow limitation with loss of expiratory flow reserve on spirometry and/or abnormal oscillometry. The increased ventilatory demand of exercise evoked additional abnormalities including expiratory flow limitation during exercise and/or small airway hyperreactivity post exercise. While some control subjects also demonstrated abnormal small airway function during resting oscillometry, there were minimal abnormalities evoked by exercise and consequently these individuals remained asymptomatic. Numerous clinical conditions were present that could have contributed variably to the small airway abnormality in individual patients with implications for determining the optimal therapeutic approach. The similarity of observations in WTC dust-exposed and diverse clinical referral populations suggests that these findings have broad relevance.
Footnotes
Provenance: Submitted article, peer reviewed.
Support statement: The study was funded by the National Institute of Occupation Safety and Health (grants 1U01OH011317 and 1U01OH010404) and from the Centers for Disease Control and Prevention (grants 200-2017-93327 and 200-2017-93427). The sponsor did not participate in the data analysis or drafting the contents of the manuscript. Funding information for this article has been deposited with the Crossref Funder Registry.
This study is registered at www.clinicaltrials.gov with identifier number NCT03089515.
Conflict of interest: None of the authors have a conflict of interest to declare.
References
- 1.Macklem PT. The physiology of small airways. Am J Respir Crit Care Med 1998; 157: S181–S183. doi: 10.1164/ajrccm.157.5.rsaa-2 [DOI] [PubMed] [Google Scholar]
- 2.Mead J. The lung's “quiet zone”. N Engl J Med 1970; 282: 1318–1319. doi: 10.1056/NEJM197006042822311 [DOI] [PubMed] [Google Scholar]
- 3.Tang FSM, Rutting S, Farrow CE, et al. . Ventilation heterogeneity and oscillometry predict asthma control improvement following step-up inhaled therapy in uncontrolled asthma. Respirology 2020; 25: 827–835. doi: 10.1111/resp.13772 [DOI] [PubMed] [Google Scholar]
- 4.Cottee AM, Seccombe LM, Thamrin C, et al. . Bronchodilator response assessed by the forced oscillation technique identifies poor asthma control with greater sensitivity than spirometry. Chest 2020; 157: 1435–1441. doi: 10.1016/j.chest.2019.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eddy RL, Westcott A, Maksym GN, et al. . Oscillometry and pulmonary magnetic resonance imaging in asthma and COPD. Physiol Rep 2019; 7: e13955. doi: 10.14814/phy2.13955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oppenheimer BW, Goldring RM, Herberg ME, et al. . Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest 2007; 132: 1275–1282. doi: 10.1378/chest.07-0913 [DOI] [PubMed] [Google Scholar]
- 7.Berger KI, Pradhan DR, Goldring RM, et al. . Distal airway dysfunction identifies pulmonary inflammation in asymptomatic smokers. ERJ Open Res 2016; 2: 00066-2016. doi: 10.1183/23120541.00066-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caplan-Shaw CE, Yee H, Rogers L, et al. . Lung pathologic findings in a local residential and working community exposed to World Trade Center dust, gas, and fumes. J Occup Environ Med 2011; 53: 981–991. doi: 10.1097/JOM.0b013e31822fff60 [DOI] [PubMed] [Google Scholar]
- 9.Friedman SM, Maslow CB, Reibman J, et al. . Case-control study of lung function in World Trade Center Health Registry area residents and workers. Am J Respir Crit Care Med 2011; 184: 582–589. doi: 10.1164/rccm.201011-1909OC [DOI] [PubMed] [Google Scholar]
- 10.Kazeros A, Zhang E, Cheng X, et al. . Systemic inflammation associated with World Trade Center dust exposures and airway abnormalities in the local community. J Occup Environ Med 2015; 57: 610–616. doi: 10.1097/JOM.0000000000000458 [DOI] [PubMed] [Google Scholar]
- 11.Johnson BD, Weisman IM, Zeballos RJ, et al. . Emerging concepts in the evaluation of ventilatory limitation during exercise: the exercise tidal flow-volume loop. Chest 1999; 116: 488–503. doi: 10.1378/chest.116.2.488 [DOI] [PubMed] [Google Scholar]
- 12.O'Donnell DE. Ventilatory limitations in chronic obstructive pulmonary disease. Med Sci Sports Exerc 2001; 33: S647–S655. doi: 10.1097/00005768-200107001-00002 [DOI] [PubMed] [Google Scholar]
- 13.O'Donnell DE, Laveneziana P. The clinical importance of dynamic lung hyperinflation in COPD. COPD 2006; 3: 219–232. doi: 10.1080/15412550600977478 [DOI] [PubMed] [Google Scholar]
- 14.Neder JA, Berton DC, Marillier M, et al. . The role of evaluating inspiratory constraints and ventilatory inefficiency in the investigation of dyspnea of unclear etiology. Respir Med 2019; 158: 6–13. doi: 10.1016/j.rmed.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 15.Di Marco F, Terraneo S, Job S, et al. . Cardiopulmonary exercise testing and second-line pulmonary function tests to detect obstructive pattern in symptomatic smokers with borderline spirometry. Respir Med 2017; 127: 7–13. doi: 10.1016/j.rmed.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 16.Stevenson NJ, Walker PP, Costello RW, et al. . Lung mechanics and dyspnea during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172: 1510–1516. doi: 10.1164/rccm.200504-595OC [DOI] [PubMed] [Google Scholar]
- 17.Stickland MK, Neder JA, Guenette JA, et al. . Using cardiopulmonary exercise testing to understand dyspnea and exercise intolerance in respiratory disease. Chest 2022; 161: 1505–1516. doi: 10.1016/j.chest.2022.01.021 [DOI] [PubMed] [Google Scholar]
- 18.Radtke T, Crook S, Kaltsakas G, et al. . ERS statement on standardisation of cardiopulmonary exercise testing in chronic lung diseases. Eur Respir Rev 2019; 28: 180101. doi: 10.1183/16000617.0101-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsons JP, Hallstrand TS, Mastronarde JG, et al. . An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med 2013; 187: 1016–1027. doi: 10.1164/rccm.201303-0437ST [DOI] [PubMed] [Google Scholar]
- 20.Pellegrino R, Viegi G, Brusasco V, et al. . Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948–968. doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 21.Graham BL, Steenbruggen I, Miller MR, et al. . Standardization of spirometry 2019 update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019; 200: e70–e88. doi: 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham BL, Brusasco V, Burgos F, et al. . 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J 2017; 49: 1600016. doi: 10.1183/13993003.00016-2016 [DOI] [PubMed] [Google Scholar]
- 23.Quanjer PH, Stanojevic S, Cole TJ, et al. . Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall GL, Filipow N, Ruppel G, et al. . Official ERS technical standard: Global Lung Function Initiative reference values for static lung volumes in individuals of European ancestry. Eur Respir J 2021; 57: 2000289. doi: 10.1183/13993003.00289-2020 [DOI] [PubMed] [Google Scholar]
- 25.Stanojevic S, Graham BL, Cooper BG, et al. . Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 2017; 50: 1700010. doi: 10.1183/13993003.00010-2017 [DOI] [PubMed] [Google Scholar]
- 26.King GG, Bates J, Berger KI, et al. . Technical standards for respiratory oscillometry. Eur Respir J 2020; 55: 1900753. doi: 10.1183/13993003.00753-2019 [DOI] [PubMed] [Google Scholar]
- 27.Berger KI, Wohlleber M, Goldring RM, et al. . Respiratory impedance measured using impulse oscillometry in a healthy urban population. ERJ Open Res 2021; 7: 00560-2020. doi: 10.1183/23120541.00560-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wasserman K, Hansen JE, Sue DY, et al. . Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. Baltimore, Lippincott Williams & Wilkins, 1999. [Google Scholar]
- 29.Chapman DG, Berend N, King GG, et al. . Increased airway closure is a determinant of airway hyperresponsiveness. Eur Respir J 2008; 32: 1563–1569. doi: 10.1183/09031936.00114007 [DOI] [PubMed] [Google Scholar]
- 30.Bates JH. Physiological mechanisms of airway hyperresponsiveness in obese asthma. Am J Respir Cell Mol Biol 2016; 54: 618–623. doi: 10.1165/rcmb.2016-0019PS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulet LP, Turcotte H, Boulet G, et al. . Deep inspiration avoidance and airway response to methacholine: influence of body mass index. Can Respir J 2005; 12: 371–376. doi: 10.1155/2005/517548 [DOI] [PubMed] [Google Scholar]
- 32.Regnard J, Baudrillard P, Salah B, et al. . Inflation of antishock trousers increases bronchial response to methacholine in healthy subjects. J Appl Physiol (1985) 1990; 68: 1528–1533. doi: 10.1152/jappl.1990.68.4.1528 [DOI] [PubMed] [Google Scholar]
- 33.Desai AG, Togias A, Schechter C, et al. . Peripheral airways dysfunction in obesity reflects increased bronchomotor tone. J Allergy Clin Immunol 2015; 135: 820–822. doi: 10.1016/j.jaci.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 34.Torchio R, Gobbi A, Gulotta C, et al. . Mechanical effects of obesity on airway responsiveness in otherwise healthy humans. J Appl Physiol (1985) 2009; 107: 408–416. doi: 10.1152/japplphysiol.00083.2009 [DOI] [PubMed] [Google Scholar]
- 35.Zerah-Lancner F, Boyer L, Rezaiguia-Delclaux S, et al. . Airway responsiveness measured by forced oscillation technique in severely obese patients, before and after bariatric surgery. J Asthma 2011; 48: 818–823. doi: 10.3109/02770903.2011.613508 [DOI] [PubMed] [Google Scholar]
- 36.Segal LN, Goldring RM, Oppenheimer BW, et al. . Disparity between proximal and distal airway reactivity during methacholine challenge. COPD 2011; 8: 145–152. doi: 10.3109/15412555.2011.560127 [DOI] [PubMed] [Google Scholar]
- 37.Berger KI, Kalish S, Shao Y, et al. . Isolated small airway reactivity during bronchoprovocation as a mechanism for respiratory symptoms in WTC dust-exposed community members. Am J Ind Med 2016; 59: 767–776. doi: 10.1002/ajim.22639 [DOI] [PubMed] [Google Scholar]
- 38.Skloot G, Schechter C, Desai A, et al. . Impaired response to deep inspiration in obesity. J Appl Physiol (1985) 2011; 111: 726–734. doi: 10.1152/japplphysiol.01155.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang LY, Cerny FJ, Kufel TJ, et al. . Simulated obesity-related changes in lung volume increases airway responsiveness in lean, nonasthmatic subjects. Chest 2006; 130: 834–840. doi: 10.1378/chest.130.3.834 [DOI] [PubMed] [Google Scholar]
- 40.Fredberg JJ, Inouye DS, Mijailovich SM, et al. . Perturbed equilibrium of myosin binding in airway smooth muscle and its implications in bronchospasm. Am J Respir Crit Care Med 1999; 159: 959–967. doi: 10.1164/ajrccm.159.3.9804060 [DOI] [PubMed] [Google Scholar]
- 41.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest 1995; 96: 2393–2403. doi: 10.1172/JCI118296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahadev S, Salome CM, Berend N, et al. . The effect of low lung volume on airway function in obesity. Respir Physiol Neurobiol 2013; 188: 192–199. doi: 10.1016/j.resp.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 43.Oppenheimer BW, Macht R, Goldring RM, et al. . Distal airway dysfunction in obese subjects corrects after bariatric surgery. Surg Obes Relat Dis 2012; 8: 582–589. doi: 10.1016/j.soard.2011.08.004 [DOI] [PubMed] [Google Scholar]