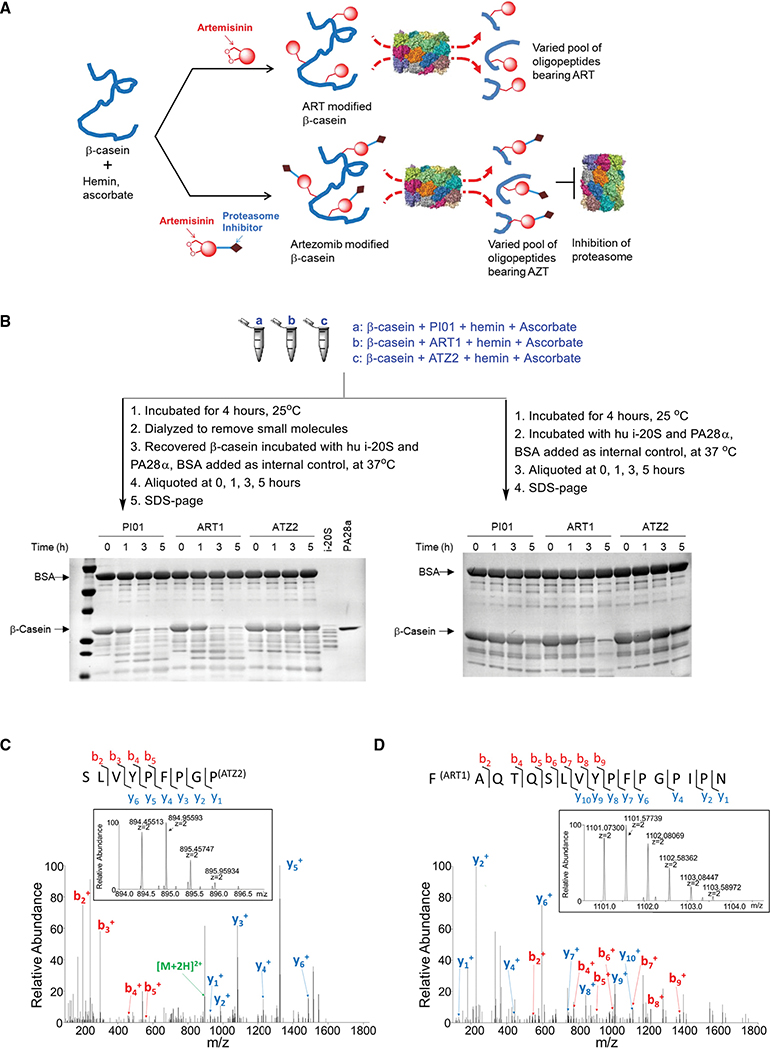
Figure 2. Mode of action of ATZ in the degradation of β-casein by 20S.
(A) Illustration of degradation of β-casein by human i-20S after incubation with ART or ATZ activated by hemin and ascorbate.
(B) Degradation of β-casein. β-casein was treated under indicated conditions (a, b, or c). (Left) after dialysis to remove the inhibitors, hemin and ascorbate, the treated β-casein was incubated with i-20S and PA28α with bovine serum albumin as an internal control. Aliquots were taken at indicated times and samples run on SDS-PAGE and stained with Coomassie blue. (Right) Without dialysis, aliquots were taken from each reaction at indicated time points and samples run on SDS-PAGE and stained with Coomassie blue. Representative images of three independent experiments for dialysis and two for non-dialysis.
(C) The tandem mass spectrometry (MS/MS) spectrum of the ATZ2-modified peptide SLVYPFPGP80. The inserted mono-isotope peak at m/z 894.45557 matches the theoretical mass of the aforementioned peptide modified by ATZ2. This peptide was not observed in PI01 treated or in ART1-treated β-casein samples through manual check.
(D) The MS/MS spectrum of the ART1 modified peptide F67AQTQSLVYPFPGPIPN. The inserted mono-isotope peak at m/z 1101.07361 matches the mass of the aforementioned peptide modified by ART1. This peptide was not observed in PI01 treated nor in ATZ2-treated β-casein samples through manual check.