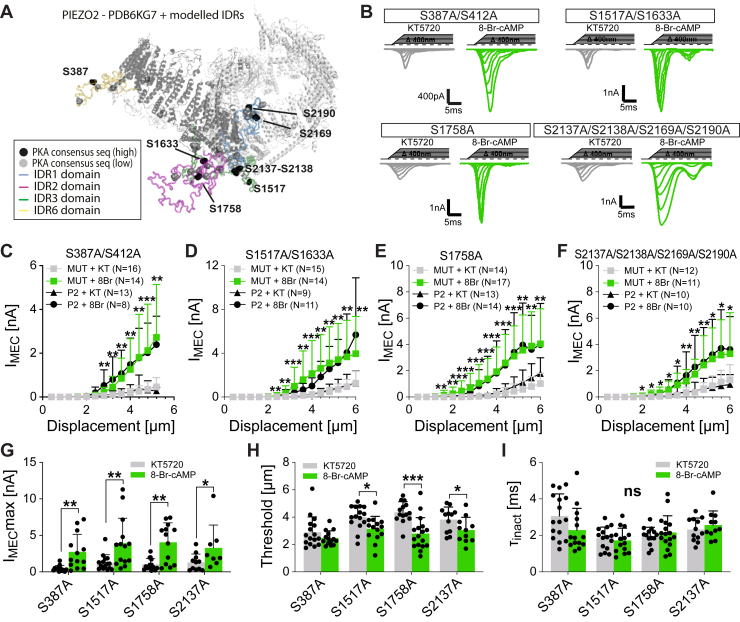
Figure 3.
Mutation of predicted PKA sites from individual intracellular domain of PIEZO2 does not prevent PKA-dependent modulation.A, side view of the mouse PIEZO2 structure (Protein Data Bank ID: 6KG7) with the modeled intracellular disordered loops that contained the predicted high (black spheres) and low (gray spheres) scores. PKA phosphorylation sites colored in the indicated color. See also Table S1. B, representative example traces from the different PIEZO2 mutants S387A/S412A (top left), the S1517A/S1633A (top right), the S1758A (bottom left), and the S2137A/S2318A/S2169A/S2190A (bottom right) in the presence of the PKA inhibitor KT5720 (gray) and the PKA activator 8-Br-cAMP (green). C–F, displacement–response curves of peak current amplitudes of PIEZO2 (black) and the different PIEZO2 PKA site mutants (gray and green) for cells treated with PKA inhibitor or activator. Data are presented as the mean ± SD. Number of cells per group is indicated in the legend. Comparison with Mann–Whitney test p < 0.05∗, p < 0.001∗∗, p < 0.0001∗∗∗, mutant + KT versus mutant + 8Br. G–I, comparison of the mean ± SD maximal current amplitude (G), mechanical activation thresholds (H), and inactivation time constants (I) of whole-cell currents from PIEZO2 mutants (same mutants as in B–F, labels only contain first mutation for better reading) treated with KT5720 (gray) or 8-Br-cAMP (green). Black circles represent individual data points. Number of cells per group are identical to those in C–F. Thresholds of the S387A/S412A mutant were compared with Mann–Whitney test p = 0.3550 and with Student's t test for all other mutants (∗p = 0.0161, S1517A; ∗∗∗p = 0.00017, S1758A; p = 0.0560, S2137A). 8-Br-cAMP, 8-bromo-cyclic-AMP.