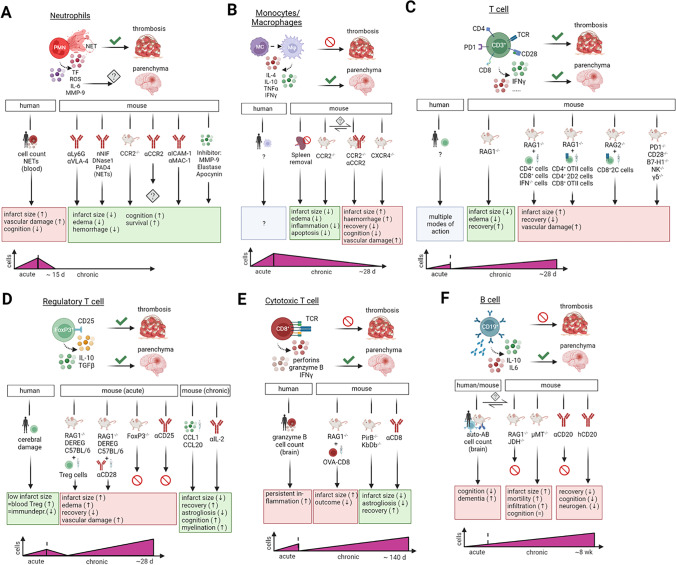
Fig. 2.
The role of immune cells in thrombosis and tissue damage after ischemic stroke. (A) During acute ischemic stroke, neutrophils promote thrombosis via release of NETs, inflammatory factors and BBB destabilizing factors. Influx of neutrophils into the brain parenchyma heavily depends on reperfusion and is still controversially discussed regarding their role in persistent tissue damage and repair. In humans, neutrophil cell count and NET propagation in the peripheral blood are connected to severe ischemic stroke. Depletion of NETs, neutrophils and blocking of adhesion to the endothelial barrier in mice correlated with improved ischemic outcome. Early inhibition of neutrophil-dependent mediators of damage e.g., MMPs and ROS result in enhanced cerebral protection. (B) The role of MM in acute ischemic stroke is not fully understood. Yet, MM are not involved in thrombosis formation, but monocytes are able to differentiate upon thrombus formation into inflammatory macrophages. Influx into the brain parenchyma afterwards might shape the inflammatory milieu found in ischemic lesions. Absence of MM, either through splenectomy or CCR2 deletion, led to less stroke burden in mice. However, CCR2 seems to be controversial since other studies showed enhancement of detrimental stroke processes. Accordingly, interrupting the chemokine axis for macrophage migration into the brain results in severe ischemic stroke. (C) General stroke experimentation with T cells uncovered that lymphocytes are involved in thrombus formation and immunological responses in the brain parenchyma. T cell deficiency led to protection against severe consequences of ischemic stroke. Genetic alteration in TCRs of T helper and cytotoxic T cells claimed TCR independent inflammation during the acute phase of ischemic stroke. (D) Knock-out of natural killer cells or conservative γδ T cells and deletion of co-stimulatory signal molecules further underlines a general independent inflammatory response after acute ischemic stroke. Tregs are an anti-inflammatory subset of T helper cells. In stroke patients, the number of circulating Treg cells positively correlates with the infarct size and absence of immunosuppression. Experimentation with mice, highlighted a detrimental role of Tregs participating in thrombus formation leading to severe ischemic damage and worse functional outcome. Adoptive transfer of Tregs into specific T cell deficient models or expansion of the Treg population was detrimental while transcription factor knock-out models and antibody depletion experiments targeting Tregs were inconclusive. In the chronic phase, however, Tregs are mandatory for recovery in the chronic phase after stroke proven in different experimental settings. (E) CTLs exert their surveillance function via TCR mediated apoptosis induction and secretion of inflammatory cytokines. CTLs are not involved in thrombosis propagation. Despite CTLs mediate detrimental effects via TCRs in the chronic phase, in the acute ischemic stroke however, CTLs enhance inflammation in a TCR-independent manner. Depletion of CD8+ T cells or use of knock-out models for TCRs of CTL led to stroke protection even in the chronic phase after ischemic insult. In patients biopsies, the number of CTL and the amount of granzyme B in brain parenchyma were in accordance with the severity of ischemic brain damage. (F) B cells are ambivalent immune cells in the pathogenesis of ischemic stroke. In thrombosis development, B cells have been extensively investigated in mice and found negligible for stroke induction. However, knock-out mouse models found no effects (RAG1-/-; JHD-/-) or beneficial effects (μMT) of B cells on brain protection processes in the acute phase of ischemic stroke. Antibody-mediated depletion of B cells controversial mediated cognitive decline and aggravated recovery in the chronic phase after stroke. Experiments in mice and humans led to contrary results. Abbreviations: μMt, inactivating mutation in the first M transmembrane exon of the μ heavy chain domain; BBB, blood-brain barrier; CCL, chemokine (C-C motif) ligand; CCR, chemokine (C-C motif) receptor; CTL, cytotoxic T cell; CXCR, chemokine (C-X-C motif) receptor; JHD, JH gene deficiency for antibody heavy chain; KbDb, gene of the major histocompatibility complex class; MC, monocyte; MM, monocytes/macrophages; MMP, matrix-metalloproteinases; Mφ, macrophage; NET, neutrophil extracellular trap; PirB, paired immunoglobulin-like receptor B; PMN, polymorphonuclear leukocytes; RAG, recombination activating gene; ROS, reactive oxygen species; TCR, T cell receptor; TF, tissue factor; Treg; regulatory T cells; VLA, very late antigen. Created with BioRender.com