Figure 3. The effect of spontaneous state fluctuations on accuracy is outcome dependent.
(A) Coefficients of a generalized linear mixed model (GLMM) fit to the mice’s choice accuracy in valid trials. Accuracy is affected by the strength of evidence, the point during the session and the outcome of the previous trial, but none of the four signals computed during the baseline explain accuracy. (B) Mean difference in accuracy after errors minus after corrects in each of the recording sessions. Triangle, median across sessions. (C) GLMM fit to accuracy computed separately after error trials. On the right, we show the distribution of a single coefficient capturing trial-to-trial fluctuations in desynchronization and firing rate simultaneously (see text). (D) Psychometric function (logistic fit, Methods) of aggregate data across sessions separately for trials with favorable ( and ) and unfavorable ( and ) baseline states after a error trials. (E, F) Same as (C, D) but for choices after a correct trial. Note that, based on the results in (E), the favorable state after a correct trial is and . (G) Schematic illustration of possible relationships between outcome, baseline cortical state and accuracy. Left, the association between state and accuracy is spurious and results from a common effect of response outcome on these two variables. Middle, epoch hypothesis (see text). An unmeasured variable with a timescale of several trials mediates both the effect of state on accuracy and the prevalence of errors. Right, response outcome gates the effect of state fluctuations (errors open the gate) on choice accuracy. (H, I) Same as (C, E) but conditioned on the outcome of the next, rather than the previous trial.
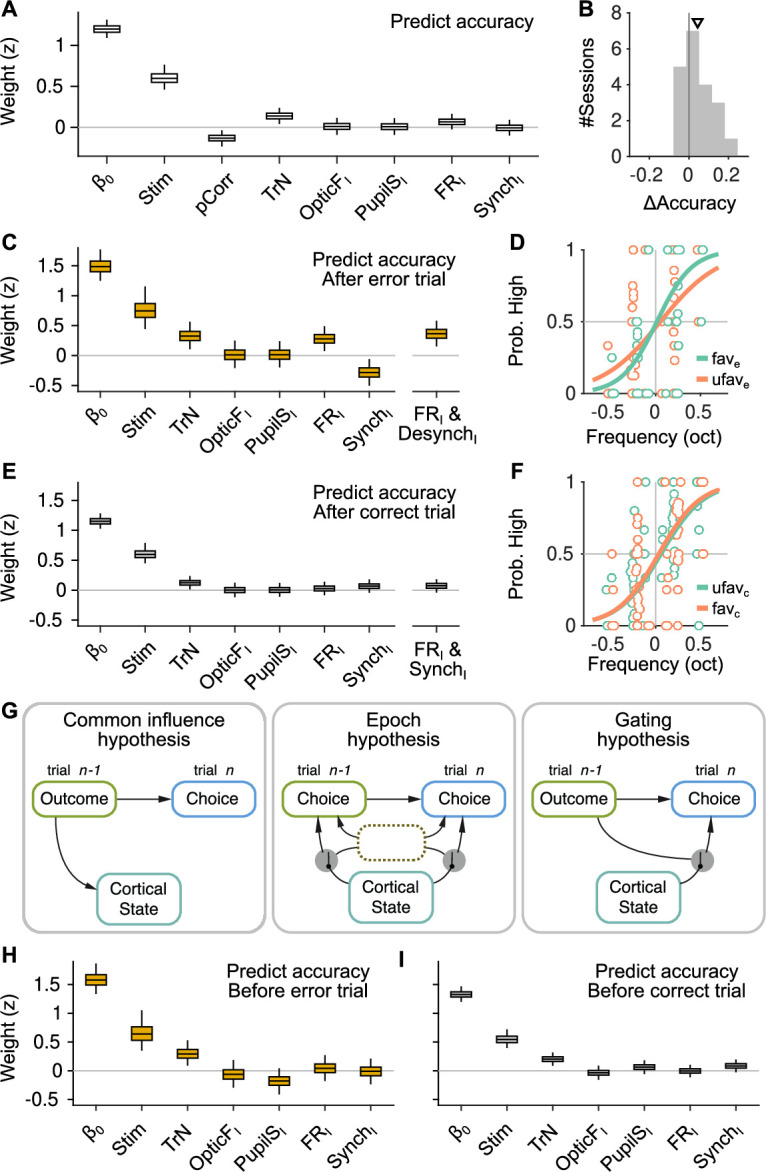
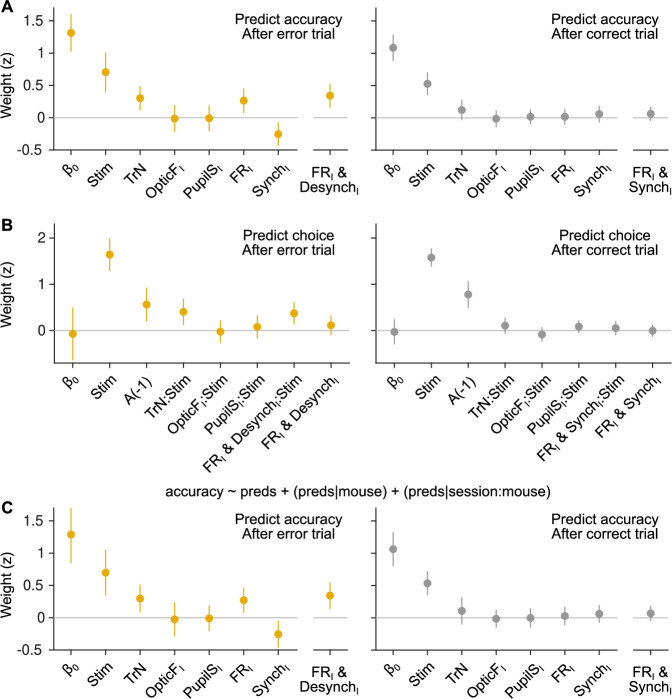
Figure 3—figure supplement 1. Robustness of the association between brain state and accuracy.
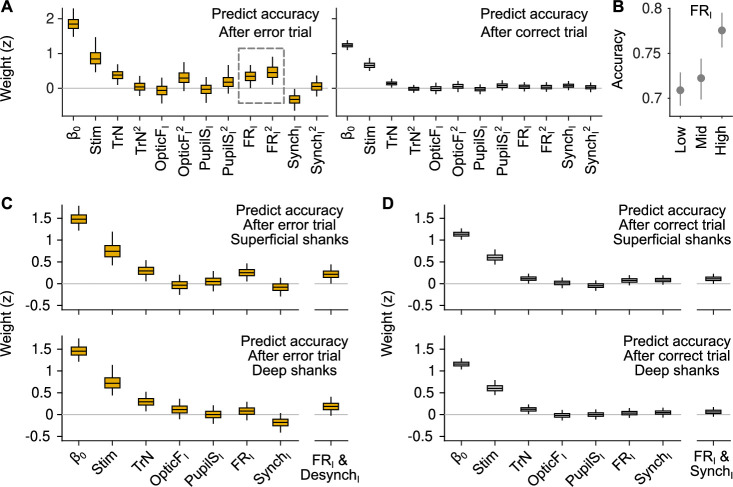
Figure 3—figure supplement 2. Generalized linear mixed model (GLMM) analysis including quadratic terms and differentially for superficial and deep recording shanks.
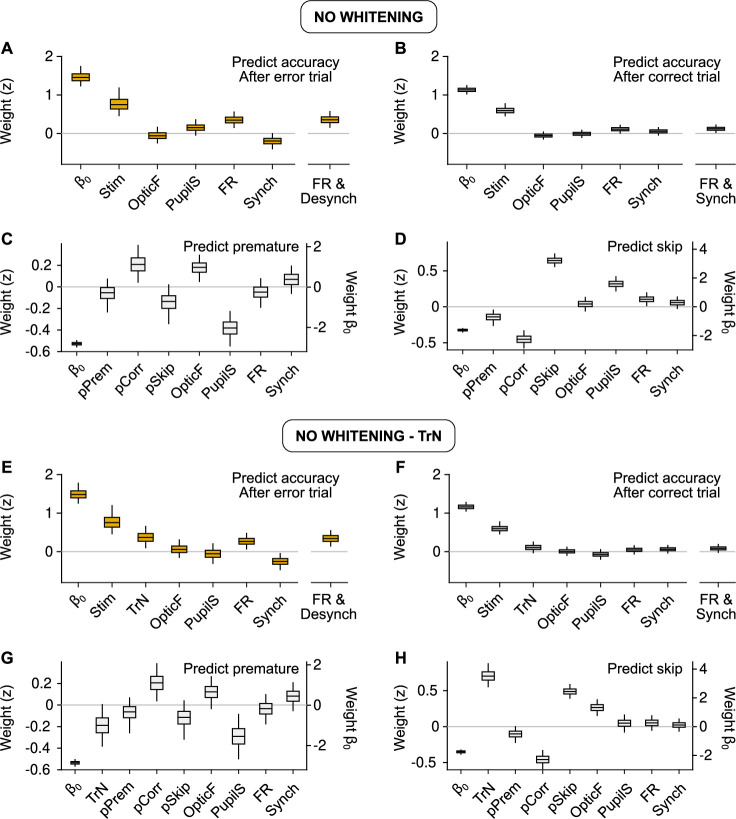
Figure 3—figure supplement 3. Robustness of the results on the effects of cortical state on accuracy.
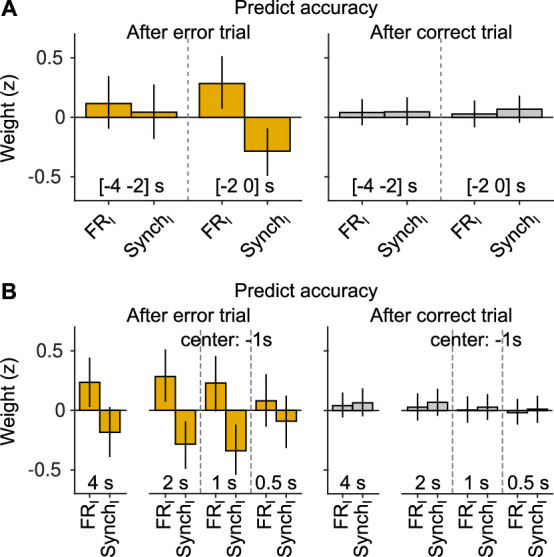
Figure 3—figure supplement 4. Lack of association between slow cortical state fluctuations and accuracy.
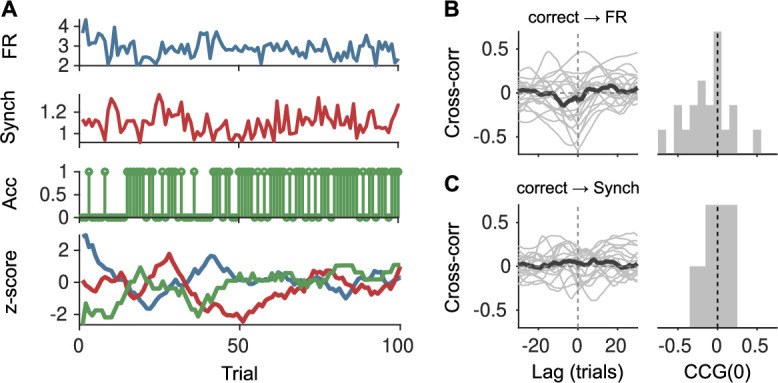
Figure 3—figure supplement 5. Behavioral predictions including slow trends.
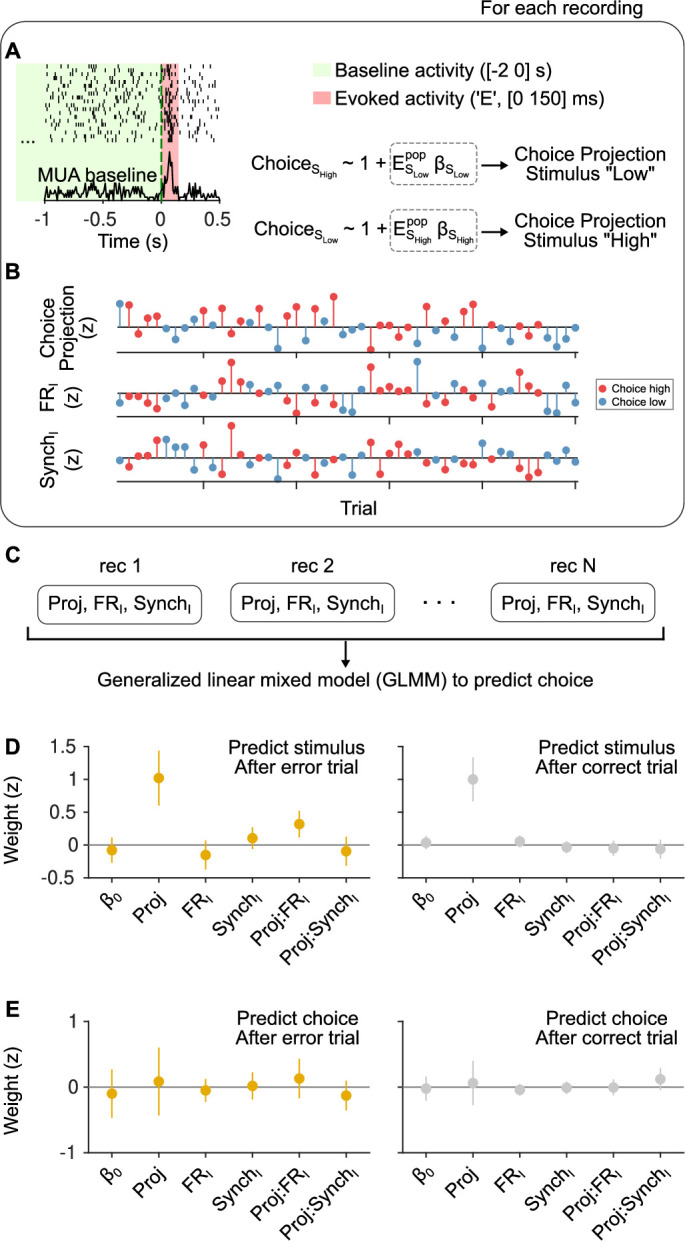
Figure 3—figure supplement 6. Outcome dependence of the effect of cortical state on stimulus and choice discriminability from evoked responses.