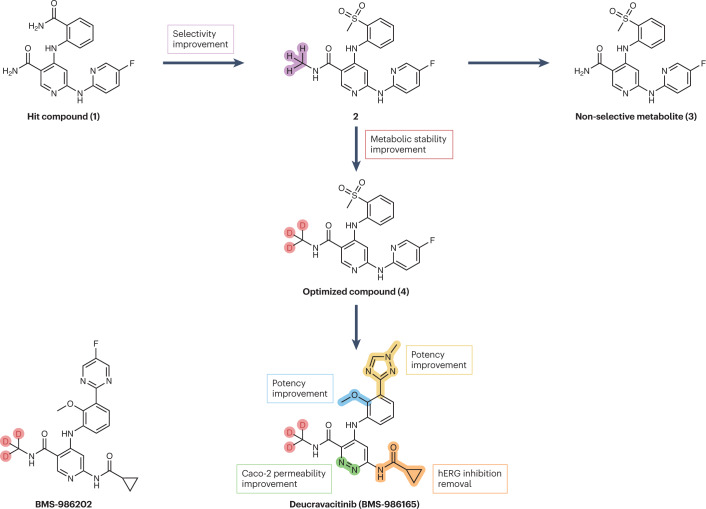
Fig. 3. Streamlined representation of the campaigns that led to the development of deucravacitinib and BMS-986202.
Starting from hit compound 1, an extensive chemical refinement led to the discovery of deucravacitinib, the first clinical pseudokinase inhibitor endowed with selectivity for tyrosine kinase 2 (TYK2) over other isoforms. First, a ‘magic’ methylation of the primary amide of compound 1 to give compound 2 boosted selectivity for TYK2 over other isoforms. Adoption of the deuterium switch approach increased the metabolic stability of compound 4, reducing the formation of non-selective metabolites such as compound 3. Subsequent late-stage optimizations afforded deucravacitinib. A parallel medicinal chemistry campaign led to the discovery of the back-up compound, BMS-986202.