Abstract
Objectives/aims
The visceral myopathies (VM) are a group of disorders characterised by poorly contractile or acontractile smooth muscle. They manifest in both the GI and GU tracts, ranging from megacystis to Prune Belly syndrome. We aimed to apply a bespoke virtual genetic panel and describe novel variants associated with this condition using whole genome sequencing data within the Genomics England 100,000 Genomes Project.
Methods
We screened the Genomics England 100,000 Genomes Project rare diseases database for patients with VM-related phenotypes. These patients were screened for sequence variants and copy number variants (CNV) in ACTG2, ACTA2, MYH11, MYLK, LMOD1, CHRM3, MYL9, FLNA and KNCMA1 by analysing whole genome sequencing data. The identified variants were analysed using variant effect predictor online tool, and any possible segregation in other family members and novel missense mutations was modelled using in silico tools. The VM cohort was also used to perform a genome-wide variant burden test in order to identify confirm gene associations in this cohort.
Results
We identified 76 patients with phenotypes consistent with a diagnosis of VM. The range of presentations included megacystis/microcolon hypoperistalsis syndrome, Prune Belly syndrome and chronic intestinal pseudo-obstruction. Of the patients in whom we identified heterozygous ACTG2 variants, 7 had likely pathogenic variants including 1 novel likely pathogenic allele. There were 4 patients in whom we identified a heterozygous MYH11 variant of uncertain significance which leads to a frameshift and a predicted protein elongation. We identified one family in whom we found a heterozygous variant of uncertain significance in KCNMA1 which in silico models predicted to be disease causing and may explain the VM phenotype seen. We did not find any CNV changes in known genes leading to VM-related disease phenotypes. In this phenotype selected cohort, ACTG2 is the largest monogenic cause of VM-related disease accounting for 9% of the cohort, supported by a variant burden test approach, which identified ACTG2 variants as the largest contributor to VM-related phenotypes.
Conclusions
VM are a group of disorders that are not easily classified and may be given different diagnostic labels depending on their phenotype. Molecular genetic analysis of these patients is valuable as it allows precise diagnosis and aids understanding of the underlying disease manifestations. We identified ACTG2 as the most frequent genetic cause of VM. We recommend a nomenclature change to ‘autosomal dominant ACTG2 visceral myopathy’ for patients with pathogenic variants in ACTG2 and associated VM phenotypes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s44162-023-00012-z.
Keywords: Visceral myopathy, Prune Belly syndrome, ACTG2, MYH11, Whole genome sequencing
Introduction
Visceral myopathies (VM) (OMIM 155310) are a group of rare congenital conditions of smooth muscle dysfunction. They are characterised by variable phenotypes, ranging from megacystis (massively distended bladder) and severe feeding intolerance secondary to intestinal dysmotility at the most severe end of the spectrum, to intermittent abdominal distension and functional intestinal obstruction at the milder end of the spectrum. These visceral myopathies have been grouped into three distinct phenotypes: megacystis-microcolon intestinal hypoperistalsis syndrome (MMIHS) (OMIM 619362, 249,210, 619,365, 619,431, 619,351), chronic intestinal pseudo-obstruction (CIPO) (OMIM 3000048) and Prune Belly syndrome (PBS) (OMIM 100100).
The clinical consequences of patients with these groups of phenotypes can be significant [17]. Those with MMIHS often require a long-term indwelling urinary catheter. This is not a permanent solution, and often, surgical intervention to create a vesicostomy is undertaken [16, 53]. The intestinal manifestations of MMIHS are often more significant, ultimately requiring surgical intervention to form a gastrostomy for feeding or ileostomy for faecal diversion. Failing these interventions, patients often require parenteral nutrition (PN), with the associated long-term risks and those of a permanent central line. Mortality in these patients is over 40% within the first year [53].
Given the rarity and broad phenotypic range of these diseases, a true epidemiologic picture is difficult to attain. CIPO is the only phenotype to have been studied in detail, with a study in Japanese babies estimating the incidence at 0.21 (male) and 0.24 (female) cases per 100,000 population [17]. As a less severe phenotype, CIPO is associated with a longer life expectancy. However, these patients are again often managed surgically with gastrostomies or surgical jejunostomies and PN [25]. One study reported survival at 10 years following initiation of PN at 75% in adults [1].
To summarise the clinical picture, VM presents with a range of phenotypes, most requiring surgical intervention for feeding, urinary or faecal diversion and with a variable mortality rate. Research into the underlying molecular genetic causes of visceral myopathy has identified several genes in which pathogenic variants have been associated with the disease. Heterozygous pathogenic variants in ACTG2 (which encodes a smooth muscle actin) were initially identified by Wangler et al. and subsequently confirmed in other clinical studies [2, 20, 32, 52]. In murine expression studies of Actg2, there was evidence of high expression in the bladder and bowel [34, 44]. Unfortunately, there have been no murine knock-out studies examining Actg2 in more detail.
Aside from ACTG2, other genes underlying visceral myopathies have been described. These include ACTA2, encoding a smooth muscle actin protein found in bladder/bowel but predominantly in vascular tissue [34]. ACTA2 variants inherited in an autosomal dominant fashion give rise to megacystis and variably, intestinal malrotation and hypoperistalsis [35]. However, smooth muscle actins encoded by this gene are also associated with vascular and ciliary smooth muscles, and therefore, aneurysms and mydriasis complete this phenotypic picture. There are several other genetic causes such as MYH11 (encoding a myosin-heavy chain) [8], which may be inherited in either an autosomal dominant or recessive manner. Phenotypes associated with MYH11 disease-causing variants include CIPO and chronic gut motility disorders inherited as an autosomal dominant pattern [5, 9]. Biallelic variants in MYH11 have been reported to cause the more severe MMIHS phenotype [51].
The remainder is inherited in an autosomal recessive manner including MYLK (encoding a myosin-light chain kinase) [11], LMOD1 (encoding leiomyodin) [13], MYL9 (encoding a regulatory myosin light chain) [37], and FLNA (encoding Filamin A — an actin-binding protein) [19]. Finally, there are several genes associated with intestinal hypoperistalsis but are due to mitochondrial disorders, rather than inherited myopathies. These include the following: EDNRB, EDN3, SOX10, SGOL1, RAD21 and L1CAM [2].
Whole genome sequencing (WGS) is becoming commonly applied to rare diseases in order to define its underlying molecular basis [48]. We utilised WGS data within the Genomics England 100,000 Genomes Project to determine the molecular basis of 76 patients recruited with a VM-related phenotype.
Methods and materials
Participants
Participants with suspected visceral myopathy disorders were recruited to the 100,000 Genomes.
Project (main programme) between 2015 and 2018 with megacystis-microcolon intestinal hypoperistalsis syndrome (MMIHS), chronic intestinal pseudo-obstruction (CIPO) or Prune Belly syndrome (PBS). All participants provided written informed consent, and the study was approved by the HRA Committee East of England Cambridge South (REC Ref 14/EE/1112).
Clinical information
Human Phenotype Ontology (HPO) terms were used to classify clinical features by organ system using a branching tree incorporating increasing levels of detail [22] (Supplementary Table S1). Patients were assigned to a disease domain, and this allowed the application of a virtual gene panel to the genomic data, based on known disease genes associated with the phenotype.
Whole genome sequencing
DNA extraction, quantification and sequencing were performed according to a national specification (Illumina TruSeq, HiSeq 2500 and HiSeq X) [48] with reads aligned to the Genome Reference Consortium Human Genome Build 38 (GRCh38) for the earlier participants recruited and GRCh38 for later participants using Isaac Genome Alignment Software. Family-based variant calling of single-nucleotide variants and insertion-deletions for chromosomes 1–22 and X was performed using the Platypus variant caller [40].
Variant analysis
Genomes were analysed in families, and variants were classified into four ‘tier’ groups according to the probability of the variant being causative [43]. Tier 1 included loss-of-function variants and de novo missense or splice region variants in genes on the virtual gene panels applied, tier 2 included missense and splice region variants in genes on the virtual gene panels applied, tier 3 included other rare variants, and a final group of unclassified variants had higher population frequency, or the segregation pattern in the family was not consistent with phenotypic information available. Virtual gene panels were chosen according to each participant’s phenotypes, using curated ‘PanelApp’ gene lists [30], to prioritise variants likely to be causative and to minimise ‘incidental findings’. There was no specific gene panel for VM; therefore, a bespoke virtual gene panel consisting of ACTG2, ACTA2, MYH11, MYLK, LMOD1, CHRM3, MYL9, FLNA and KCNMA1 was applied. Tiers 1–3 gene variants were accessed from the Main Programme v11_2019-11–28. All tiered gene variants had passed in-house Genomics England quality control. Sequence variants were also prioritised using Exomiser [42]. Variants classified as pathogenic, likely pathogenic or pathogenic/likely pathogenic were identified using ClinVar [24] for GRCh38 and GRCh37 and compared against tier 1–3 variants using bedtools intersect (https://bedtools.readthedocs.io). If parental data were available, a segregation of alleles and phenotypes was performed to determine if alleles were inherited or de novo.
Clinical review
Tier 1 and tier 2 variants, the top ten prioritised variants by Exomiser and ClinVar pathogenic/likely pathogenic variants, were reviewed by a clinical geneticist and classified by ACMG criteria [39], using information from gnomAD [21], Ensembl [56], VarSome [23], OMIM and review of the literature. Feedback from the GMC laboratories and clinical teams was incorporated when available. Variant quality was checked using the Integrative Genomics Viewer (IGV) [41]. The molecular diagnosis was described as ‘definite’, ‘probable’ or ‘possible’ based on the ACMG classification of the variant(s), the inheritance pattern and the clinical fit between the patient’s HPO terms and the reported clinical phenotypes for the genetic variant. The contribution was described as ‘full’ or ‘partial’ depending on whether the whole phenotype or only one aspect could be explained by the identified variant(s) from the VM virtual gene panel.
Genome-wide variant burden tests
Individuals with a VM-related phenotype were contrasted with a control group of individuals. Controls were selected from the rare diseases cohort of individuals and were (i) not probands, (ii) labelled ‘unaffected’ and had no HPO terms that included any of the words or partial words: ‘bladder’, ‘ureter’, ‘urin’, ‘renal’ or ‘muscl’. A merged control vcf file was produced using bcftools. The genes and gene locations considered were from Ensembl gene coordinates version 96 for GRCh38. These genes were extracted using tabix and annotated using variant effect predictor (VEP) [31]. The number of variants per gene per individual that was of (i) of frequency < 0.001 and (ii) could have high and moderate impact consequences (frameshift, missense and stop variant annotations) was counted using a custom R script, and, for each gene, the number of individuals with at least one qualifying variant was compared between cases and controls using a Fisher’s exact test.
Copy number variant analysis
Copy number depth was calculated using bcftools depth every 20 bases for the panel genes and for control genes in three sets (20 neighbouring, 20 more distant on the same chromosome and 20 on different autosomes). The average depth for each individual and for each gene in the panel was scaled by the average depth on the control genes for each control set. A copy number was then called based on the relative depth of each gene to the control set. Differences between calls based on neighbouring control sets and far control sets are investigated further for larger deletions or duplications [7, 18].
Spatial modelling
The spatial structure of KCNMA1 proteins was modelled and visualised with AlphaFold-2 and PyMOL 2.3 software to determine protein folding of missense variant of interest [47].
Phenotype enrichment analysis
In an unbiased approach, the application of a gene variant analysis of ACTG2 to WGS data from all probands and relatives (n = 65,507, including all phenotypes) in the Genomics England 100,000 Genomes Project Rare Disease project who carried out. Results were annotated and then filtered for probands alone and missense variants in ACTG2 with an AF < 1% (in any gnomAD population) and a CADD score > 20 or synonymous ACTG2 variants with an AF < 1% (in any gnomAD population). The phenotypes of probands with ACTG2 variants (either missense or synonymous as a control group) were analysed for associated ICD10 terms. The prevalence of each ICD10 term that was encountered was calculated and phenotype enrichment ratio calculated. Fisher’s exact test was computed for each retained analysed phenotype and phenotypes ranked according to p-value of statistical significant enrichment.
Results
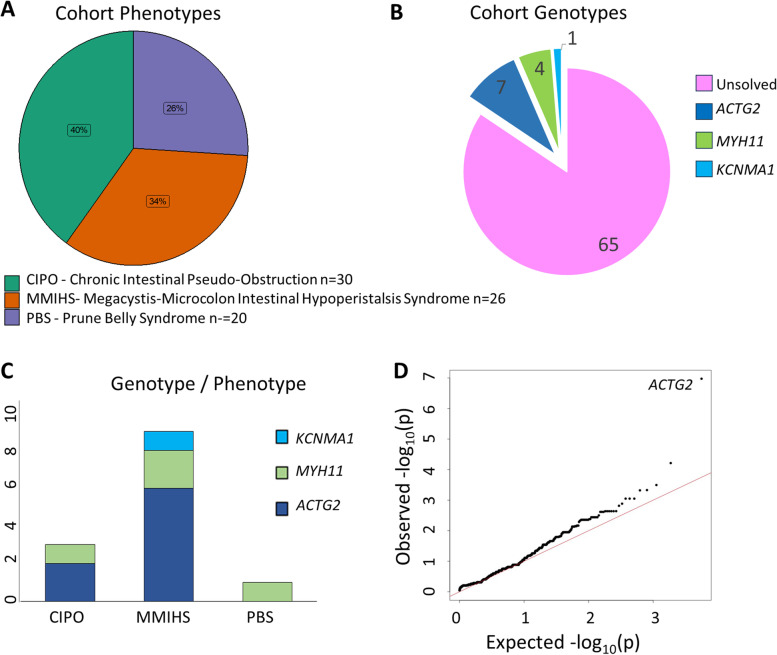
Overall, there were 76 patients in the Genomics England 100,000 Genomes Project rare disease cohort with phenotypes that represented VM phenotypes (n = 30 with CIPO, n = 26 with MMIHS and n = 20 with PBS) (Fig. 1A). Application of the custom VM gene panel demonstrated no participants with pathogenic or likely pathogenic variants in ACTA2, LMOD1 or MYL9. No rare CNVs within the virtual gene panel were detected.
Fig. 1.
Visceral myopathy cohort and genetic variants identified in this study. A Cohort of 76 patients with visceral myopathy phenotypes including sub-groups CIPO, MMIHS and PBS. B Patients genotype with pathogenic/likely pathogenic variants in ACTG2; VUS in MYH11 and VUS in KCNMA1. C Genotype–phenotype correlations within the identified patients. D Quantile–quantile plot for rare variants associated with visceral myopathy phenotypes following genome-wide variant burden test. Shown are empirically observed quantiles of rare gene effects (AF < 0.001) (y-axis) as a function of quantiles expected from a normal distribution with the same mean and variance as the empirical distribution (x-axis). Variants in ACTG2 were the only statistically significant finding (p = 1.1 × 10−.7)
In total, we identified 11 VM patients with rare ACTG2 variants, 6 with rare MYH11 variants, 3 with rare MYLK variants, 1 with a rare CHRM3 variant, 3 with rare FLNA variants and 1 with a rare KCNMA1 variant of uncertain significance. In order to determine the pathogenicity, an in silico analysis of each of these genetic findings was performed. For each patient, the clinical phenotypes and any genetic segregation analysis where available were also reviewed to determine which patients with VM-associated phenotypes could be genetically solved.
Patients with rare ACTG2 alleles
Heterozygous mutations in ACTG2 are associated with VM phenotypes including MMIHS type 5 (OMIM 619431) and visceral myopathy type 1 (OMIM 155310). We identified 11 VM patients carrying rare ACTG2 alleles, 9 of whom had severe early onset VM phenotypes. Of these 11 patients, 5 were determined to be genetically ‘solved’ by Genomics England with a diagnostic variant in ACTG2 reported in the Genomics England exit questionnaire. These variants included known pathogenic missense alleles p.Arg40His (patient 1), p.Arg178Leu (patient 3), p.Arg257Cys (patient 4, de novo) and p.Arg257His (patient 5, de novo; patient 6, de novo). In another VM patient (patient 2), we identified a previously reported and known pathogenic allele p.Arg148Cys, inherited from their affected father allowing this patient to be genetically solved (Table 1 and Supplementary Fig. S1). For the remaining 5 patients with VM phenotypes, who were unsolved by Genomics England as segregation data for potential causative variants was lacking, we identified novel heterozygous ACTG2 variants (Table 1). Pathogenicity scores suggested that one of these alleles was likely pathogenic and disease causing (c.338C > T; p.Pro113Leu, patient 9), although family segregation data is incomplete, whilst 4 were classified as variants of uncertain significance (VUS). Heterozygous pathogenic and likely pathogenic variants (all missense alleles) in ACTG2 therefore solved 7 out of 76 (9.2%) VM patients and were associated with phenotypes related to CIPO and MMIHS (Fig. 1B, C).
Table 1.
Patient phenotypes and ACTG2 alleles identified following WGS
| Participant | Ethnicity | Phenotype | ACTG2 nucleotide change | ACTG2 amino acid change | ACMG classification | SIFT score | PolyPhen-2 score | Allele frequency | Solved by GEL | Comments | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 — female | White, British | Visceral myopathy, abdominal distension, abdominal pain, foetal megacystis, gastrointestinal dysmotility, constipation, intestinal malrotation, intestinal pseudo-obstruction | c.119G > A | p.(Arg40His) |
Pathogenic (11 points) P, PP5, PM1, PM5, PP3, PM2 |
Deleterious (0.02) | Probably damaging (0.991) | 0.00001 (PAGE study [4] | Y |
rs587777386 Unaffected mother wild type genotype; unaffected father, no genotype |
[2, 32, 36, 38, 52, 55] |
| 2 — male | White, European | Intestinal pseudo-obstruction, gastrointestinal dysmotility, constipation | c.442C > T | p.(Arg148Cys) | Pathogenic (10 points) P, PM5, PP3, PM1, PP5, PM2 | Deleterious (0.02) | Probably damaging (0.989) |
0.000004 (Gnomad) |
Y |
rs587777383 AD pattern, affected father also has pathogenic allele |
[15, 26, 38, 39] |
| 3 — female | White, European | Pseudo-obstruction, microcolon, megacystis, hypoperistalsis, feeding difficulties | c.533G > T | p.(Arg178Leu) |
Likely pathogenic (17 points) LP, PS3, PM5, PP3, PP5, PM2, PP2 |
Deleterious (0) | Probably damaging (0.775) | N/A | Y |
rs587777384 Unaffected mother, wild-type genotype; unaffected father no genotype |
[6, 33, 46, 49, 52] |
| 4 — male | N/A | Intestinal pseudo-obstruction, myopathy, feeding difficulties, gastrointestinal dysmotility, megacystis, congenital hydronephrosis | c.769C > T | p.(Arg257Cys) |
Pathogenic (16 points) P, PP5, PP3, PM5, PM2, PP2 |
Deleterious (0.04) | Probably damaging (0.989 |
0.00008 (PAGE study [4]) |
Y |
rs587777387 De novo, both parents unaffected and wild type |
[2, 12, 28, 29, 32, 33, 36, 38, 39, 46, 49, 52, 54] |
| 5 — female | N/A | Intestinal pseudo-obstruction, myopathy, feeding difficulties, gastrointestinal dysmotility, megacystis, constipation, neuromuscular dysfunction of bladder | c.770G > A | p.(Arg257His) |
Pathogenic (16 points) P, PP5, PP3, PM5, PM2, PP2 |
Deleterious (0.04) | Probably damaging (0.989) | N/A | Y |
rs797044959 De novo both parents unaffected and wild type |
[33, 46, 49, 52] |
| 6 — male | N/A | Intestinal pseudo-obstruction, myopathy, feeding difficulties, gastrointestinal dysmotility | c.770G > A | p.(Arg257His) |
Pathogenic (16 points) P, PP5, PP3, PM5, PM2, PP2 |
Deleterious (0.04) | Probably damaging (0.989) | N/A | Y |
rs797044959 De novo both parents unaffected and wild type |
[33, 46, 49, 52] |
| 7 — male | White, British | Intestinal pseudo-obstruction, microcolon, megacystis | c.226A > T | p.(Ile76Phe) | Uncertain significance (3 points) VUS, PP3, PM2, PP2 | Deleterious (0) | Probably damaging (0.961) | N/A | N |
Novel unaffected mother, wild-type genotype; unaffected father no genotype |
Novel |
| 8 — female | Asian | CAKUT, bladder extrophy | c.287G > A | p.(Arg96His) |
Uncertain significance (4 points) VUS, PP3, PM2, PP2 |
Deleterious (0.04) | Benign (0.219) | 9.02 × 10−4 (Genomics England) | N |
rs1363649764 — VUS Unaffected parents, no genotype |
Novel |
| 9 — female | White, British | Megacystis, hydroureter, hydronephrosis, intestinal malrotation, constipation, microcolon | c.338C > T | p.(Pro113Leu) |
Likely pathogenic (6 points) LP, PP3, PM1, PM5, PM2 |
Deleterious (0.01) | Probably damaging (1) | N/A | N |
Novel unaffected mother, wild-type genotype; unaffected father, no genotype |
Novel |
| 10 — female | White, British | Cystic kidney disease, hypertension, recurrent UTIs | c.386A > G | p.(Asn129Ser) |
Uncertain significance (2 points) VUS, PM2, PP2 |
Tolerated (0.14) | Benign (0.04) | 0.0045 (Genomics England) | N |
rs77469596 Father affected, no genotype; unaffected mother, no genotype |
Novel |
| 11 — male | White, British | Prune Belly syndrome, congenital hydronephrosis | c.850A > G | p.(Met284Val) |
Uncertain significance (4 points) VUS, PP3, PM2, PP2 |
Deleterious (0.01) | Benign (0.382) | N/A | N |
Novel unaffected mother, no genotype; unaffected father, no genotype |
Novel |
ACTG2 NM_001199893. SIFT values of between 0 and 0.05 are predicted to affect protein function. PolyPhen-2 values between 0.85 and 1.0 are confidently predicted to be damaging, values between 0.15 and 1.0 range are possibly damaging and values between 0.0 and 0.15 are predicted to be benign
Patients with rare MYH11 alleles
Both heterozygous variants [5, 9] and biallelic variants [51] in MYH11 are typically associated with VM phenotypes that include autosomal recessive MMIHS type 2 (OMIM 619351) and autosomal dominant visceral myopathy type 2 (OMIM 619350). Biallelic variants typically cause more severe clinical phenotypes, suggesting a spectrum of disease according to inheritance pattern. In our cohort, we identified 6 VM patients with heterozygous MYH11 variants, none of whom was previously genetically solved by Genomics England. There was one participant (patient 16), with Prune Belly syndrome with a novel MYH11 heterozygous missense variant (p.(Lys1141Gln)), classified as a VUS and unlikely on its own to explain this severe phenotype. There were four patients (patients 12, 13, 14, 15), with variable clinical phenotypes of VM, who had the same heterozygous MYH11 c.5819del; p.(Pro1940Hisfs*91) frameshift allele (Table 2 and Supplementary Fig. S2). This variant is classified as a VUS and was found in patient 15 with a mild phenotype with features of constipation, colostomy and urinary retention (Table 2). This MYH11 frameshift allele is identical to the MYH11 allele reported by Gilbert et al. in a three-generation family with five affected individuals, with the proband diagnosed with constipation in infancy and followed but infantile pseudo-obstruction at the age of 11 years. Anal manometry measurements performed at 20 years of age showed findings consistent with a smooth muscle myopathy [9]. Interestingly, of 2 of the 4 patients with this identical allele also presented with intestinal pseudo-obstruction. The same allele was also reported by Dong et al. in a three-generation family with 7 affected with a CIPO phenotype, including 3 individuals with bowel complications including bowel obstruction, rectal prolapse and malrotation of the bowel [5]. Together, these cases provide an accumulation of evidence for a role in VM phenotypes.
Table 2.
Patient phenotypes and MYH11 alleles identified following WGS
| Participant | Ethnicity | Phenotype | MYH11 nucleotide change |
MYH11 amino acid change |
ACMG classification | SIFT score | PolyPhen-2 score | Allele frequency | Solved by GEL | Comments | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 11 — male | White, British | PBS, congenital hydronephrosis | c.3766A > C | p.(Lys1256Gln) |
Likely benign (− 2 points) BP4, BP1, PM2 |
Tolerated (0.51) | Probably damaging (0.93) | 0.000299 (gnomAD) | N |
rs149241435 Mother and father unaffected, no genotype available |
Novel |
| 12 — male | White, British | CAKUT, megacystis, hydronephrosis, recurrent UTIs | c.5819del | p.(Pro1940Hisfs*91) | Uncertain significance (2 points) | Deleterious (0.02) | Probably damaging (0.991) |
6.82 × 10−5 (gnomAD) |
N |
rs747392139 Mother unaffected, genotype wild type; father unaffected, no genotype available |
[5, 9] |
| 13 — female | White, British | Intestinal pseudo-obstruction, myopathy | c.5819del | p.(Pro1940Hisfs*91) | Uncertain significance (2 points) | Deleterious (0.02) | Probably damaging (0.991) |
6.82 × 10−5 (gnomAD) |
N |
rs747392139 Mother unaffected, no genotype available; father affected, no genotype available |
[5, 9] |
| 14 — male | White, British | Intestinal pseudo-obstruction, megacystis, myopathy, constipation, gastrointestinal dysmotility | c.5819del | p.(Pro1940Hisfs*91) | Uncertain significance (2 points) | Deleterious (0.02) | Probably damaging (0.991) |
6.82 × 10−5 (gnomAD) |
N |
rs747392139 Mother affected, no genotype available; father unaffected, no genotype available |
[5, 9] |
| 15 — male | White, British | PBS, retention of urine, colostomy | c.5819del | p.(Pro1940Hisfs*91) | Uncertain significance (2 points) | Deleterious (0.02) | Probably damaging (0.991) |
6.82 × 10−5 (gnomAD) |
N |
rs747392139 Mother and father unaffected, no genotype available |
[5, 9] |
| 16 — male | White, British | Hypertension, chronic kidney disease | c.3421A > C | p.(Lys1141Gln) |
Uncertain significance (0 point) PM2, BP1 |
Deleterious (0.01) | Probably damaging (0.88) | N/A | N |
rs797045725 Mother and father unaffected, no genotype available |
Novel |
MYH11 NM_022844. SIFT values of between 0 and 0.05 are predicted to affect protein function. PolyPhen-2 values between 0.85 and 1.0 are confidently predicted to be damaging, values between 0.15 and 1.0 range are possibly damaging and values between 0.0 and 0.15 are predicted to be benign
Patient 11, with a Prune Belly syndrome phenotype, had a MYH11 likely benign allele in addition to the identified ACTG2 VUS allele c.850A > G; p.(Met284Val) which is insufficient evidence to solve this case. In total, heterozygous predicted loss-of-function variants in MYH11 but classified as VUS by ACMG criteria (Table 2) were found in 4 (patients 12, 13, 14, 15) out of 76 (5%) patients with VM phenotypes (Fig. 1B).
Patients with rare MYLK alleles
Biallelic variants in MYLK are associated with VM phenotypes that include MMIHS type 1 (OMIM 249210). In our VM cohort, we identified 3 VM patients with variable phenotypes with heterozygous MYLK variants (Supplementary Table S2). The first variant was a synonymous variant, and the second was a missense variant. The SpliceAI results of the synonymous variant were low and is therefore likely compensated, and the allele is likely benign. The missense variant was predicted as likely benign. Given that known MYLK genetic variants associated with VM are all biallelic (homozygous frameshift mutations), these variants are unlikely to be pathogenic and causative of the disease phenotype in these VM cases.
Patients with rare CHRM3 alleles
Biallelic variants in CHRM3 are associated with Prune Belly syndrome (OMIM 100100). We identified one VM patient (patient 20) with a heterozygous missense CHRM3 variant, classified as a VUS (Supplementary Table S3). This heterozygous variant alone is unlikely to be pathogenic and causative of the disease phenotype in this case.
Patients with rare FLNA alleles
Pathogenic variants in FLNA cause an X-linked recessive form of neuronal intestinal pseudo-obstruction (OMIM 300048). We identified one male participant with FLNA variants. This participant (patient 21) had 3 FLNA rare variants, 2 splice region variants and 1 synonymous variant. The splice region variants identified were c.5741-8C > T and c.5289 + 4C > T and predicted to be of low impact. The SpliceAI results of the synonymous variant were also low and is therefore likely compensated suggesting these variants were unlikely to be causative of the disease phenotype in this case (Supplementary Table S4).
Genome-wide variant burden test
We performed a genome-wide variant burden test using our cohort of VM patients as our target population and with 918 selected controls. There was only one highly significant gene identified (ACTG2 (p = 1.1 × 10−7)) from the assembled alleles (Fig. 1D and Supplementary Table S5). The finding of ACTG2 as the most significant associated gene with VM phenotypes is consistent with our findings from the application of a virtual gene panel for this cohort. It also suggests that other genetic causes of VM within this cohort are rare.
Phenotype enrichment analysis
Up to this point, our investigations had been limited to a well-defined cohort of patients with defined VM phenotypes. In an attempt to perform an unbiased analysis of disease phenotypes associated with ACTG2 variants, we performed gene variant extraction on the whole rare disease cohort within the Genomics England 100,000 disease project. We found 37 missense carriers (an AF < 1% in any gnomAD population and a CADD score > 20) and 164 synonymous variant carriers (with an AF < 1% in any gnomAD population). The phenotypes of these individuals with ACTG2 variants (either rare and deleterious missense or synonymous as a control group) were analysed for associated ICD10 terms. In total, 405 statistical tests were performed, and statistical significance was adjusted for multiple testing with statistical significance p < 1.2 × 10−4 (0.05/405). Only ICD term Q64 ‘Other congenital malformations of urinary system’ reached phenome-wide statistical significance in the cohort, although several gastrointestinal phenotypes (potentially compatible with manifestations of visceral myopathy) reached nominal significance (Supplementary Table S6).
Patients with rare KCNMA1 alleles
Heterozygous pathogenic variants in KCNMA1 cause Liang-Wang syndrome (OMIM 618729), a severe neurological disorder that may include severe global developmental delay, craniofacial dysmorphism and visceral and connective tissue abnormalities [27]. Biallelic variant in KCNMA1 is associated with cerebellar atrophy, developmental delay and seizures (OMIM 617643) [45]. KCNMA1 encodes for a calcium-activated potassium channel and has a role in the modulation of vascular smooth muscle potassium channels [50]. Recently, a de novo heterozygous variant in KCNMA1 (c.1123G > A; p.Gly375Arg) was reported in a child with absent abdominal musculature [3] which prompted us to examine our VM cohort for variants in this gene.
We identified one VM patient with early onset intestinal pseudo-obstruction, megacystis, constipation, feeding difficulties and gastrointestinal dysmotility with a heterozygous KCNMA1 rare variant (Table 3 and Supplementary Fig. S3). This missense variant p.(Arg1128Gln) has a low SIFT score (0.07) and high PolyPhen score (0.991) and is classified as a VUS using the ACMG criteria. The unaffected father was wild type for the allele, and the mother (also affected with joint hypermobility, poor wound healing and constipation) had the same heterozygous variant, suggesting an autosomal dominant pattern of inheritance of a VM-related phenotype. This variant therefore remains an interesting and novel candidate variant for the VM phenotypes exhibited in this family. The allele frequency within the 100,000 genomes rare disease project was 0.00002. The gnomAD allele frequency, a ‘healthy control population’, is 0.000012, suggesting that either this allele is not fully penetrant or can cause subclinical phenotypes. For missense alleles in KCNMA1, gnomAD data shows a Z score of 5.06, indicating some intolerance to variation. Modelling of this variant showed a predicted new abnormal bond to the adjacent on lysine at position 1127 which provides some evidence to support its pathogenicity (Fig. 2).
Table 3.
Patient phenotypes and KCNMA1 alleles identified following WGS
| Participant | Ethnicity | Phenotype | KCNMA1 genetic variant | Amino acid change | ACMG classification | SIFT | PolyPhen-2 | Allele frequency | Solved by GEL | Comments | Reference or novel |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 — female | White, British | Intestinal pseudo-obstruction, megacystis, constipation, feeding difficulties, gastrointestinal dysmotility | c.3383G > A | p. (Arg1128Gln) |
Uncertain significance (1 point) PM2 supporting PP3 supporting BP1 supporting |
Deleterious (0.02) |
Probably damaging (0.999) |
0.000012 (gnomAD) | N |
rs200141207 Mother affected with same genotype; father unaffected, no genotype available |
Novel |
KCNMA1 NM_002247.4 SIFT A value of between 0 and 0.05 is predicted to affect protein function. PolyPhen-2 value between 0.85 and 1.0 is confidently predicted to be damaging; values between 0.15 and 1.0 range are possibly damaging. Values between 0.0 and 0.15 are predicted to be benign
Fig. 2.
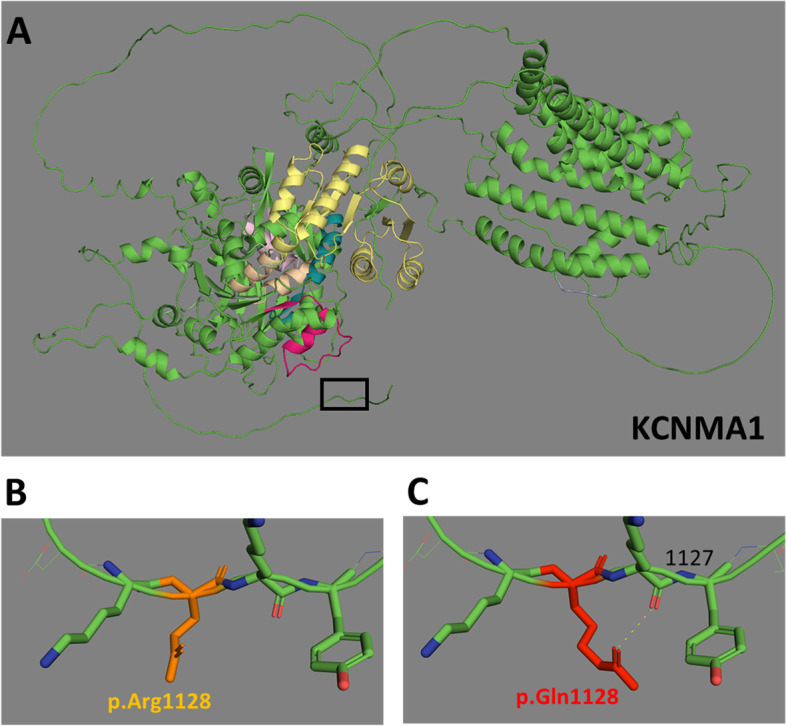
AlphaFold-2 models of KCNMA1 missense variant. A Low-resolution model of KCNMA1 protein structure (NP_002238.2) with region containing amino acid position 1128 boxed. B Wild-type KCNMA1 (NP_002238.2) p.(Arg1128) and C. missense KCNMA1 p.(Arg1128Gln), with likely bond to adjacent leucine (position 1127)
Discussion
We present the first examination of the Genomics England rare disease database for the VM group of diseases, a diverse set of phenotypes with a wide spectrum of clinical severity. We identified known pathogenic and novel variants in ACTG2 and MYH11 likely accounting for a total of 12 cases of VM-related phenotypes. Also, in this set of known VM-causing genes, we identified several VUS that require further evaluation. In KCNMA1, a candidate gene for VM, we identified a novel missense variant that appeared to segregate with VM phenotypes in an autosomal dominant manner. This variant requires further investigation and validation however. For the genetically solved cases in ACTG2, all variants were rare heterozygous missense variants with high PolyPhen and low SIFT scores (i.e. predicted highly pathogenic) in patients with VM, with no other explanatory variants. The 9 ACTG2 variants were also classed as pathogenic using ACMG criteria. For the genetically solved cases in MYH11, all 4 patients shared a heterozygous pathogenic frameshift mutation previously associated with VM [5] and intestinal pseudo-obstruction [9] in an autosomal dominant pattern.
The genome-wide variant burden test which was performed on the VM cohort identified ACTG2 as the major gene implicated and is consistent with our gene panel findings for this cohort. As confirmatory evidence, ACTG2 was also recently identified as the most strongly associated gene in patients with gastrointestinal disorders using a Bayesian genetic association method [10]. The extreme variability in disease phenotypes suggests that VM-related disorders are likely to be multifactorial disorders with genetic as well as epigenetic and environmental factors playing a role. Dissecting out each of these contributing factors will be important for the understanding of these conditions [14].
Unbiased phenotypic enrichment analysis did not suggest additional phenotypes for ACTG2 beyond kidney and gastrointestinal tract involvement. This does not exclude completely that ACTG2 variants could contribute to milder gastrointestinal phenotypes such as malabsorption and ileus, and further work in less severe disease cohorts is warranted.
This study’s strength lies in the systematic nature of the WGS database examination and the database itself. The Genomics England database is a large national database comprising ~ 85,000 patients with rare disease and/or cancer, all of whom have had WGS [43]. We have applied rigorous searches to this dataset to ensure we identify any potential patient with VM and any rare, potentially pathogenic variants in the genes associated with VM. The main weakness in this study is the small number of patients with VM in the Genomics England database (n = 76) and the difficulty in identifying these patients from the recorded HPO and phenotypic descriptors. A general weakness of any study taking advantage of Genomic England data is the lack of detailed phenotypic information with no direct access to the participants’ clinicians or their imaging data.
ACTG2, the leading cause of VM [2], was the most highly represented gene in our analysis, a result confirmed by our genome-wide variant burden test study within the Genomics England cohort. The extent of ACTG2 variation in the context of VM has previously been described [2], and patients with arginine substitutions in particular suffer more severe phenotypes. Assia Batzir et al. have also compiled a list of all known ACTG2 variants associated with VM, all are heterozygous missense variants [2]. With this wealth of supporting data concerning ACTG2 variants in association with VM phenotype,s we recommend a change in the nomenclature for these patients to ‘autosomal dominant ACTG2 visceral myopathy’.
We identified missense ACTG2 variants that were not clearly pathogenic. The alleles p.(Arg96His) and p.(Asn129Ser)) were associated with CAKUT and recurrent UTI phenotypes, respectively, but may possibly represent milder ACTG2 clinical phenotypes. A previously reported family, where a mother of two siblings with microcolon, did not have bladder/bowel involvement, but did have postpartum uterine atony hint at this phenotypic diversity. All three family members had the same heterozygous ACTG2 variant p.(Arg178Cys) [2].
The second most common cause of VM we identified was MYH11. In the literature, these are predominantly autosomal recessive cases with biallelic variants; however, autosomal dominant forms have been reported [5, 9]. We identified four patients with variable clinical phenotypes of VM, who had the same heterozygous p.(Pro1940Hisfs*91) frameshift allele, suggesting this allele may be an autosomal dominant cause of VM. We also identified a patient with Prune Belly syndrome with a novel heterozygous variant in MYH11 (p.(Lys1141Gln)), which is classified as a VUS. Unfortunately, this patient was a singleton, and no segregation data is therefore available, and further inference about the pathogenicity of this variant is premature. Functional studies are needed to determine if these potential novel variants we have identified are indeed pathogenic.
The overall solve rate of this diverse phenotypically heterogeneous cohort was low at around 9%. Many of the known VM-associated genes, including ACTA2, MYLK, LMOD1, CHRM3, MYL9 and FLNA did not have variants that explained the patients’ phenotypes. This implies that for VM patients, there may be many more alternative underlying genetic causes to be identified. As we have shown, variants in MYH11 and KCNMA1 represent a possible genetic cause for this disease phenotype, but other yet to be discovered contributory genes will need to be identified.
Conclusions
VM phenotypes are diverse but include severe, life-threatening disorders caused by smooth muscle weakness in the bladder, bowel and uterus. We present molecular genetic variants including novel alleles in ACTG2 and MYH11 associated with VM phenotypes that allow a precise molecular diagnosis to be reached. In our VM cohort, ACTG2 mutations were the leading cause of VM, and we recommend a nomenclature change to autosomal dominant ACTG2 visceral myopathy for such cases.
Supplementary Information
Additional file 1: Figure S1. IGV visualisation of genetic variants in ACTG2. Figure S2. IGV visualisation of genetic variants in MYH11. Figure S3. IGV visualisation of genetic variants in KCNMA1. Table S1. OMIM IDs and HPO Terms used for phenotypic searches. Table S2. Patient phenotypes and MYLK alleles identified following WGS. Table S3. Patient phenotypes and CHRM3 alleles identified following WGS. Table S4. Patient phenotypes and FLNA alleles identified following WGS. Table S5. Genome wide variant burden test in a cohort of patients with visceral myopathy phenotypes. Table S6. Top 20 ICD phenotypes enriched in carriers of rare predicted pathogenic ACTG2 missense variants (compared to carriers of rare synonymous variant carriers) in the Genomics England 100,000 Genomes project.
Acknowledgements
This research was made possible through access to the data and findings generated by the 100,000 Genomes Project. The 100,000 Genomes Project is managed by Genomics England Limited (a wholly owned company of the Department of Health and Social Care). The 100,000 Genomes Project is funded by the National Institute for Health Research and NHS England. The Wellcome Trust, Cancer Research UK and the Medical Research Council have also funded research infrastructure. The 100,000 Genomes Project uses data provided by participants and their families and collected by the National Health Service as part of their care and support.
Authors’ contributions
JAS conceived the manuscript. RMG wrote the first draft, and SO and EO were major contributors in writing the manuscript. Data analysis was performed by RN, MBG, HM and IW. All authors read and approved the final manuscript.
Funding
R. M. G. is supported by the National Institute for Health Research as an Academic Clinical Fellow. EO is supported by the Early Postdoc Mobility Stipendium of the Swiss National Science Foundation and Kidney Research UK. RN is supported by the Barbour Foundation. MBG is supported by Kidney Research UK. HM is supported by the MRC. JAS is supported by Kidney Research UK and the Northern Counties Kidney Research Fund.
Availability of data and materials
Data sharing is available through the Genomics England Research Consortium.
Declarations
Ethics approval and consent to participate
All participants provided written informed consent, and the study was approved by the HRA Committee East of England Cambridge South (REC Ref. 14/EE/1112).
Consent for publication
Not applicable, data is anonymised.
Competing interests
Professor John Sayer is a co-author of this study and editorial board member of the journal. He was not involved in handling this manuscript during the review process. The rest of the authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Amiot A, Joly F, Alves A, Panis Y, Bouhnik Y, Messing B. Long-term outcome of chronic intestinal pseudo-obstruction adult patients requiring home parenteral nutrition. Am J Gastroenterol. 2009;104(5):1262–1270. doi: 10.1038/ajg.2009.58. [DOI] [PubMed] [Google Scholar]
- 2.Assia Batzir N, Kishor Bhagwat P, Larson A, Coban Akdemir Z, Bagłaj M, Bofferding L, . . . Wangler M.F. Recurrent arginine substitutions in the ACTG2 gene are the primary driver of disease burden and severity in visceral myopathy. Hum Mutat. 2020; 41(3): 641–654. 10.1002/humu.23960 [DOI] [PMC free article] [PubMed]
- 3.Bockenhauer D, Mushtaq I, Faravelli F. Absent abdominal musculature in a girl. Kidney Int. 2022;101(4):833. doi: 10.1016/j.kint.2021.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Carss KJ, Hillman SC, Parthiban V, McMullan DJ, Maher ER, Kilby MD, Hurles ME. Exome sequencing improves genetic diagnosis of structural fetal abnormalities revealed by ultrasound. Hum Mol Genet. 2014;23(12):3269–3277. doi: 10.1093/hmg/ddu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong W, Baldwin C, Choi J, Milunsky JM, Zhang J, Bilguvar K, . . . Milunsky A. Identification of a dominant MYH11 causal variant in chronic intestinal pseudo-obstruction: results of whole-exome sequencing. Clin Genet. 2019; 96(5): 473–477. 10.1111/cge.13617 [DOI] [PubMed]
- 6.Farwell KD, Shahmirzadi L, El-Khechen D, Powis Z, Chao EC, Tippin Davis B, . . . Tang S. Enhanced utility of family-centered diagnostic exome sequencing with inheritance model-based analysis: results from 500 unselected families with undiagnosed genetic conditions. Genet Med. 2015; 17(7): 578–586. 10.1038/gim.2014.154 [DOI] [PubMed]
- 7.Flanagan SE, Patch AM, Ellard S. Using SIFT and PolyPhen to predict loss-of-function and gain-of-function mutations. Genet Test Mol Biomarkers. 2010;14(4):533–537. doi: 10.1089/gtmb.2010.0036. [DOI] [PubMed] [Google Scholar]
- 8.Gauthier J, Bencheikh OA, B., Hamdan, F.F., Harrison, S.M., Baker, L.A., Couture, F., … Soucy, J.F. A homozygous loss-of-function variant in MYH11 in a case with megacystis-microcolon-intestinal hypoperistalsis syndrome. Eur J Hum Genet. 2015;23(9):1266–1268. doi: 10.1038/ejhg.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert MA, Schultz-Rogers L, Rajagopalan R, Grochowski CM, Wilkins BJ, Biswas S, . . . Spinner NB. Protein-elongating mutations in MYH11 are implicated in a dominantly inherited smooth muscle dysmotility syndrome with severe esophageal, gastric, and intestinal disease. Hum Mutat. 2020; 41(5): 973–982. 10.1002/humu.23986 [DOI] [PubMed]
- 10.Greene D, Pirri D, Frudd K, Sackey E, Al-Owain M, Giese APJ, . . . Turro E. Genetic association analysis of 77,539 genomes reveals rare disease etiologies. Nat Med. 2023; 29(3): 679–688. 10.1038/s41591-023-02211-z [DOI] [PMC free article] [PubMed]
- 11.Halim D, Brosens E, Muller F, Wangler MF, Beaudet AL, Lupski JR, . . . Alves MM. Loss-of-function variants in MYLK cause recessive megacystis microcolon intestinal hypoperistalsis syndrome. Am J Hum Genet. 2017; 101(1): 123–129. 10.1016/j.ajhg.2017.05.011 [DOI] [PMC free article] [PubMed]
- 12.Halim D, Hofstra RM, Signorile L, Verdijk RM, van der Werf CS, Sribudiani Y, . . . Alves MM. ACTG2 variants impair actin polymerization in sporadic megacystis microcolon intestinal hypoperistalsis syndrome. Hum Mol Genet. 2016; 25(3): 571–583. 10.1093/hmg/ddv497 [DOI] [PubMed]
- 13.Halim D, Wilson MP, Oliver D, Brosens E, Verheij JB, Han Y, . . . Miano JM. Loss of LMOD1 impairs smooth muscle cytocontractility and causes megacystis microcolon intestinal hypoperistalsis syndrome in humans and mice. Proc Natl Acad Sci U S A. 2017; 114(13): E2739-e2747. 10.1073/pnas.1620507114 [DOI] [PMC free article] [PubMed]
- 14.Hashmi SK, Ceron RH, Heuckeroth RO. Visceral myopathy: clinical syndromes, genetics, pathophysiology, and fall of the cytoskeleton. Am J Physiol Gastrointest Liver Physiol. 2021;320(6):G919–g935. doi: 10.1152/ajpgi.00066.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holla OL, Bock G, Busk OL, Isfoss BL. Familial visceral myopathy diagnosed by exome sequencing of a patient with chronic intestinal pseudo-obstruction. Endoscopy. 2014;46(6):533–537. doi: 10.1055/s-0034-1365142. [DOI] [PubMed] [Google Scholar]
- 16.Hugar LA, Chaudhry R, Fuller TW, Cannon GM, Schneck FX, Ost MC, Stephany HA. Urologic phenotype and patterns of care in patients with megacystis microcolon intestinal hypoperistalsis syndrome presenting to a major pediatric transplantation center. Urology. 2018;119:127–132. doi: 10.1016/j.urology.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Iida H, Ohkubo H, Inamori M, Nakajima A, Sato H. Epidemiology and clinical experience of chronic intestinal pseudo-obstruction in Japan: a nationwide epidemiologic survey. J Epidemiol. 2013;23(4):288–294. doi: 10.2188/jea.je20120173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaganathan K, Kyriazopoulou Panagiotopoulou S, McRae JF, Darbandi SF, Knowles D, Li YI, . . . Farh KK. Predicting splicing from primary sequence with deep learning. Cell. 2019; 176(3): 535–548.e524. 10.1016/j.cell.2018.12.015 [DOI] [PubMed]
- 19.Kapur RP, Robertson SP, Hannibal MC, Finn LS, Morgan T, van Kogelenberg M, Loren DJ. Diffuse abnormal layering of small intestinal smooth muscle is present in patients with FLNA mutations and x-linked intestinal pseudo-obstruction. Am J Surg Pathol. 2010;34(10):1528–1543. doi: 10.1097/PAS.0b013e3181f0ae47. [DOI] [PubMed] [Google Scholar]
- 20.Klar J, Raykova D, Gustafson E, Tóthová I, Ameur A, Wanders A, Dahl N. Phenotypic expansion of visceral myopathy associated with ACTG2 tandem base substitution. Eur J Hum Genet. 2015;23(12):1679–1683. doi: 10.1038/ejhg.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch L. Exploring human genomic diversity with gnomAD. Nat Rev Genet. 2020;21(8):448. doi: 10.1038/s41576-020-0255-7. [DOI] [PubMed] [Google Scholar]
- 22.Köhler S, Doelken SC, Mungall CJ, Bauer S, Firth HV, Bailleul-Forestier I, . . . Robinson PN. The human phenotype ontology project: linking molecular biology and disease through phenotype data. Nucleic Acids Res. 2014; 42(Database issue): D966–974. 10.1093/nar/gkt1026 [DOI] [PMC free article] [PubMed]
- 23.Kopanos C, Tsiolkas V, Kouris A, Chapple CE, Albarca Aguilera M, Meyer R, Massouras A. VarSome: the human genomic variant search engine. Bioinformatics. 2019;35(11):1978–1980. doi: 10.1093/bioinformatics/bty897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, . . . Maglott DR. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016; 44(D1): D862–868. 10.1093/nar/gkv1222 [DOI] [PMC free article] [PubMed]
- 25.Lehmann S, Ferrie S, Carey S. Nutrition management in patients with chronic gastrointestinal motility disorders: a systematic literature review. Nutr Clin Pract. 2020;35(2):219–230. doi: 10.1002/ncp.10273. [DOI] [PubMed] [Google Scholar]
- 26.Lehtonen HJ, Sipponen T, Tojkander S, Karikoski R, Järvinen H, Laing NG, . . . Tuupanen S. Segregation of a missense variant in enteric smooth muscle actin γ-2 with autosomal dominant familial visceral myopathy. Gastroenterology. 2012; 143(6): 1482–1491.e1483. 10.1053/j.gastro.2012.08.045 [DOI] [PubMed]
- 27.Liang L, Li X, Moutton S, Schrier Vergano SA, Cogné B, Saint-Martin A, . . . Wang QK. De novo loss-of-function KCNMA1 variants are associated with a new multiple malformation syndrome and a broad spectrum of developmental and neurological phenotypes. Hum Mol Genet. 2019; 28(17): 2937–2951. 10.1093/hmg/ddz117 [DOI] [PMC free article] [PubMed]
- 28.Lu W, Xiao Y, Huang J, Tao Y, Yan W, Lu L, . . . Cai W. Mutation in Actin γ-2 responsible for megacystis microcolon intestinal hypoperistalsis syndrome in 4 Chinese patients. J Pediatr Gastroenterol Nutr. 2016; 63(6): 624–626. 10.1097/mpg.0000000000001204 [DOI] [PubMed]
- 29.Lupski JR, Gonzaga-Jauregui C, Yang Y, Bainbridge MN, Jhangiani S, Buhay CJ, . . . Gibbs RA. Exome sequencing resolves apparent incidental findings and reveals further complexity of SH3TC2 variant alleles causing Charcot-Marie-Tooth neuropathy. Genome Med. 2013; 5(6): 57. 10.1186/gm461 [DOI] [PMC free article] [PubMed]
- 30.Martin AR, Williams E, Foulger RE, Leigh S, Daugherty LC, Niblock O, . . . McDonagh EM. PanelApp crowdsources expert knowledge to establish consensus diagnostic gene panels. Nat Genet. 2019; 51(11): 1560–1565. 10.1038/s41588-019-0528-2 [DOI] [PubMed]
- 31.Martin FJ, Amode MR, Aneja A, Austine-Orimoloye O, Azov AG, Barnes I, . . . Flicek P. Ensembl 2023. Nucleic Acids Res. 2023; 51(D1): D933-d941. 10.1093/nar/gkac958 [DOI] [PMC free article] [PubMed]
- 32.Matera I, Bordo D, Di Duca M, Lerone M, Santamaria G, Pongiglione M, . . . Ceccherini I. Novel ACTG2 variants disclose allelic heterogeneity and bi-allelic inheritance in pediatric chronic intestinal pseudo-obstruction. Clin Genet. 2021; 99(3): 430–436. 10.1111/cge.13895 [DOI] [PubMed]
- 33.Matera I, Rusmini M, Guo Y, Lerone M, Li J, Zhang J, . . . Ceccherini I. Variants of the ACTG2 gene correlate with degree of severity and presence of megacystis in chronic intestinal pseudo-obstruction. Eur J Hum Genet. 2016; 24(8), 1211–1215. 10.1038/ejhg.2015.275 [DOI] [PMC free article] [PubMed]
- 34.McHugh KM, Crawford K, Lessard JL. A comprehensive analysis of the developmental and tissue-specific expression of the isoactin multigene family in the rat. Dev Biol. 1991;148(2):442–458. doi: 10.1016/0012-1606(91)90263-3. [DOI] [PubMed] [Google Scholar]
- 35.Milewicz DM, Østergaard JR, Ala-Kokko LM, Khan N, Grange DK, Mendoza-Londono R, . . . Regalado ES. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet A. 2010; 152a(10): 2437–2443. 10.1002/ajmg.a.33657 [DOI] [PMC free article] [PubMed]
- 36.Milunsky A, Baldwin C, Zhang X, Primack D, Curnow A, Milunsky J. Diagnosis of chronic intestinal pseudo-obstruction and megacystis by sequencing the ACTG2 gene. J Pediatr Gastroenterol Nutr. 2017;65(4):384–387. doi: 10.1097/mpg.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno CA, Metze K, Lomazi EA, Bertola DR, Barbosa RH, Cosentino V, . . . Cavalcanti DP. Visceral myopathy: clinical and molecular survey of a cohort of seven new patients and state of the art of overlapping phenotypes. Am J Med Genet A. 2016; 170(11): 2965–2974. 10.1002/ajmg.a.37857 [DOI] [PMC free article] [PubMed]
- 38.Ravenscroft G, Pannell S, O'Grady G, Ong R, Ee HC, Faiz F, . . . Laing NG. Variants in ACTG2 underlie a substantial number of Australasian patients with primary chronic intestinal pseudo-obstruction. Neurogastroenterol Motil. 2018; 30(9): e13371. 10.1111/nmo.13371 [DOI] [PubMed]
- 39.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, . . . Rehm HL. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17(5): 405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed]
- 40.Rimmer A, Phan H, Mathieson I, Iqbal Z, Twigg SRF, Wilkie AOM, . . . Lunter G. Integrating mapping-, assembly- and haplotype-based approaches for calling variants in clinical sequencing applications. Nat Genet. 2014; 46(8): 912–918. 10.1038/ng.3036 [DOI] [PMC free article] [PubMed]
- 41.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smedley D, Jacobsen JO, Jäger M, Köhler S, Holtgrewe M, Schubach M, . . . Robinson PN. Next-generation diagnostics and disease-gene discovery with the Exomiser. Nat Protoc. 2015; 10(12): 2004–2015. 10.1038/nprot.2015.124 [DOI] [PMC free article] [PubMed]
- 43.Smedley D, Smith KR, Martin A, Thomas EA, McDonagh EM, Cipriani V, . . . Caulfield M. 100,000 genomes pilot on rare-disease diagnosis in health care - preliminary report. N Engl J Med. 2021; 385(20): 1868–1880. 10.1056/NEJMoa2035790 [DOI] [PMC free article] [PubMed]
- 44.Szucsik JC, Lessard JL. Cloning and sequence analysis of the mouse smooth muscle gamma-enteric actin gene. Genomics. 1995;28(2):154–162. doi: 10.1006/geno.1995.1126. [DOI] [PubMed] [Google Scholar]
- 45.Tabarki B, AlMajhad N, AlHashem A, Shaheen R, Alkuraya FS. Homozygous KCNMA1 mutation as a cause of cerebellar atrophy, developmental delay and seizures. Hum Genet. 2016;135(11):1295–1298. doi: 10.1007/s00439-016-1726-y. [DOI] [PubMed] [Google Scholar]
- 46.Thorson W, Diaz-Horta O, Foster J, 2nd, Spiliopoulos M, Quintero R, Farooq A, . . . Tekin M. De novo ACTG2 mutations cause congenital distended bladder, microcolon, and intestinal hypoperistalsis. Hum Genet. 2014; 133(6): 737–742. 10.1007/s00439-013-1406-0 [DOI] [PubMed]
- 47.Tunyasuvunakool K, Adler J, Wu Z, Green T, Zielinski M, Žídek A, . . . Hassabis D. Highly accurate protein structure prediction for the human proteome. Nature. 2021; 596(7873): 590–596. 10.1038/s41586-021-03828-1 [DOI] [PMC free article] [PubMed]
- 48.Turro E, Astle WJ, Megy K, Gräf S, Greene D, Shamardina O, . . . Ouwehand WH. Whole-genome sequencing of patients with rare diseases in a National Health System. Nature. 2020; 583(7814): 96–102. 10.1038/s41586-020-2434-2 [DOI] [PMC free article] [PubMed]
- 49.Tuzovic L, Tang S, Miller RS, Rohena L, Shahmirzadi L, Gonzalez K, . . . Anyane-Yeboa K. New insights into the genetics of fetal megacystis: ACTG2 mutations, encoding γ-2 smooth muscle actin in megacystis microcolon intestinal hypoperistalsis syndrome (Berdon syndrome). Fetal Diagn Ther. 2015; 38(4): 296–306. 10.1159/000381638 [DOI] [PubMed]
- 50.Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, . . . Latorre R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 1999; 285(5435): 1929–1931. 10.1126/science.285.5435.1929 [DOI] [PubMed]
- 51.Wang Q, Zhang J, Wang H, Feng Q, Luo F, Xie J. Compound heterozygous variants in MYH11 underlie autosomal recessive megacystis-microcolon-intestinal hypoperistalsis syndrome in a Chinese family. J Hum Genet. 2019;64(11):1067–1073. doi: 10.1038/s10038-019-0651-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wangler MF, Gonzaga-Jauregui C, Gambin T, Penney S, Moss T, Chopra A, . . . Beaudet A. Heterozygous de novo and inherited mutations in the smooth muscle actin (ACTG2) gene underlie megacystis-microcolon-intestinal hypoperistalsis syndrome. PLoS Genet. 2014; 10(3): e1004258. 10.1371/journal.pgen.1004258 [DOI] [PMC free article] [PubMed]
- 53.Wymer KM, Anderson BB, Wilkens AA, Gundeti MS. Megacystis microcolon intestinal hypoperistalsis syndrome: case series and updated review of the literature with an emphasis on urologic management. J Pediatr Surg. 2016;51(9):1565–1573. doi: 10.1016/j.jpedsurg.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 54.Yang Y, Muzny DM, Reid JG, Bainbridge MN, Willis A, Ward PA, . . . Eng CM. Clinical whole-exome sequencing for the diagnosis of Mendelian disorders. N Engl J Med. 2013; 369(16): 1502–1511. 10.1056/NEJMoa1306555 [DOI] [PMC free article] [PubMed]
- 55.Yang Y, Muzny DM, Xia F, Niu Z, Person R, Ding Y, . . . Eng CM. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014; 312(18): 1870–1879. 10.1001/jama.2014.14601 [DOI] [PMC free article] [PubMed]
- 56.Yates AD, Achuthan P, Akanni W, Allen J, Allen J, Alvarez-Jarreta J, . . . Flicek P. Ensembl 2020. Nucleic Acids Res. 2020; 48(D1): D682-d688. 10.1093/nar/gkz966 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. IGV visualisation of genetic variants in ACTG2. Figure S2. IGV visualisation of genetic variants in MYH11. Figure S3. IGV visualisation of genetic variants in KCNMA1. Table S1. OMIM IDs and HPO Terms used for phenotypic searches. Table S2. Patient phenotypes and MYLK alleles identified following WGS. Table S3. Patient phenotypes and CHRM3 alleles identified following WGS. Table S4. Patient phenotypes and FLNA alleles identified following WGS. Table S5. Genome wide variant burden test in a cohort of patients with visceral myopathy phenotypes. Table S6. Top 20 ICD phenotypes enriched in carriers of rare predicted pathogenic ACTG2 missense variants (compared to carriers of rare synonymous variant carriers) in the Genomics England 100,000 Genomes project.
Data Availability Statement
Data sharing is available through the Genomics England Research Consortium.