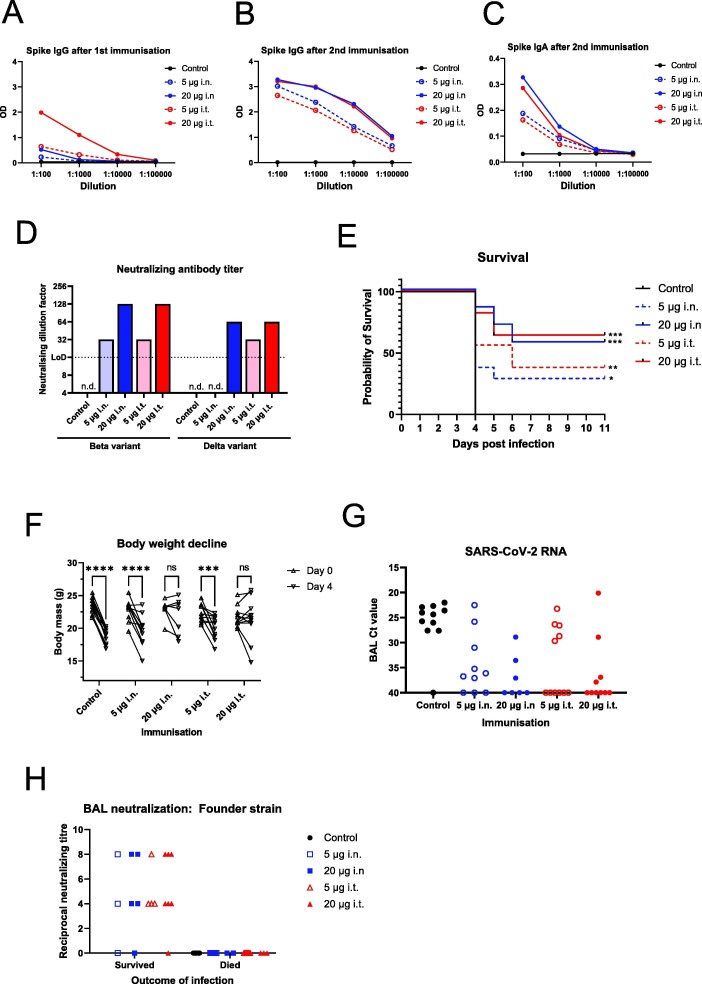
Fig. 2.
Dose-dependent protection from vaccination with a high infectious virus dose challenge model. A) Female hACE2 mice were immunised twice with combination alpha and beta variant Spike S1 by the indicated routes of administration. Fourteen days after the 1st immunisation, blood samples from vaccinated mice were taken and the serum diluted and analysed for IgG against the Founder strain Spike protein by ELISA. The average optical density (OD) for each vaccinated group is plotted for each dilution. B) As A, except with serum samples obtained 14 days after the 2nd immunisation. C) As B, except anti-Spike S1 IgA was analysed. D) Neutralizing activity of pre-challenge serum against variants of concern was assessed. Pre-challenge sera were pooled for each vaccinated group and tested for neutralizing activity against beta variant of concern and delta variant of concern at a starting dilution of 1-in-16 (LoD). D) Immunised mice were infected intranasally with 1.05x106 TCID SARS-CoV-2 isolate SARS-CoV-2/01/human/2020/SWE 14 days after the second immunisation. Health was monitored and animals were euthanized after losing > 20% of their body mass or when other symptoms became severe. Alternatively, surviving animals were euthanized at 11 dpi. Figure shows a Kaplan-Meier survival curve of the control and vaccinated groups. Vaccinated groups are labelled with total dose of Spike S1 and the route of administration; subcutaneous; i.n., intranasal; i.t., intratracheal. All groups included 11 animals each except high dose i.n. group that included 7 animals. Statistical analysis by log-rank (Mantel-Cox) test where * indicates P < 0.05, ** indicates P < 0.01 *** indicates P < 0.001 in a comparison with control animals. F) Absolute body mass of individual mice at day 0 and day 4 post infection for each group. Statistical analysis by two-way ANOVA using Sidak’s multiple comparison test, ns indicates P > 0.05, *** indicates P < 0.001, **** indicates P < 0.0001. G) SARS-CoV-2 RNA levels in BAL fluid at termination. Equal amounts of BAL fluid for each mouse was subjected to RNA extraction and SARS-CoV-2 E gene RT-qPCR. Ct values are plotted for each vaccine group.H) Neutralizing antibodies from BAL fluid at termination. 1 mL of BAL fluid for each mouse was collected at the day of euthanasia. BAL was then diluted beginning at 1-in-4 (LoD) and tested for neutralization of SARS- CoV-2 infection (founder variant) of Vero E6 cells. Results for each individual are plotted and further divided into mice that survived to day 11 (Protected) and those that were euthanized early due to symptoms (Not protected).