Figure 6.
Structural characterization of the trimer-preferring mAb S370.7
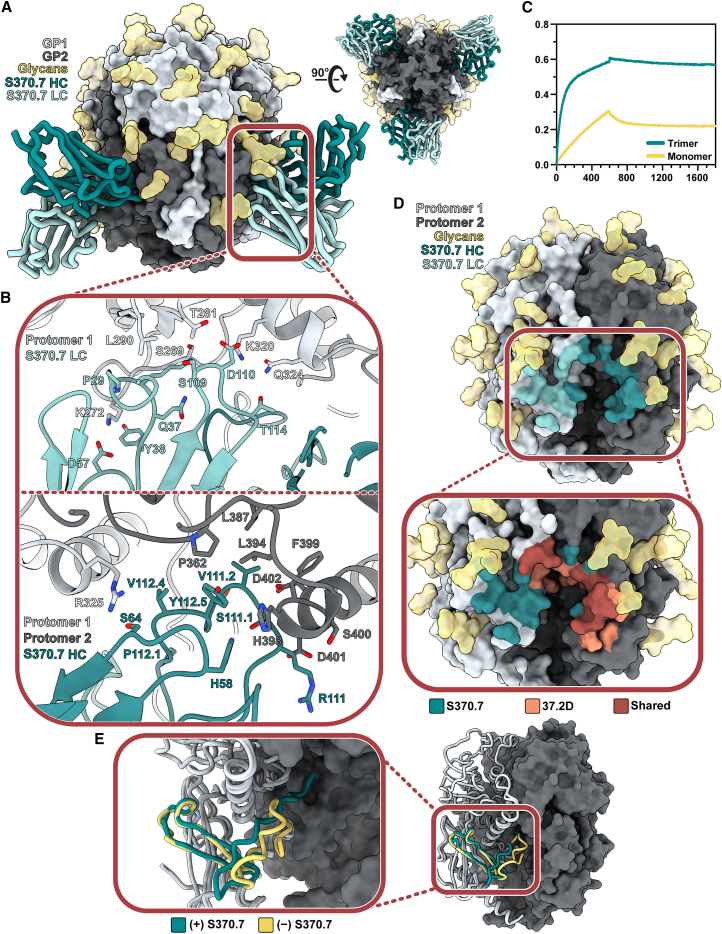
(A) Atomic model of LIV GPC (gray) bound to S370.7 Fab (teal) determined by cryo-EM.
(B) Key interactions between S370.7 LC (top) and HC (bottom) residues with GPC. More detailed information can be found in Table S4.
(C) BLI sensorgram showing the binding profile of immobilized S370.7 IgG to GPC trimer or GPC monomer in equal protomer concentrations. Presented data indicate representative curves from three technical replicates.
(D) S370.7 Ab footprint. HC interactions are shown in dark teal and LC interactions in light teal. Inset image shows the overlap and distinctions with known GPC-B-binding NAb 37.2D. Further comparisons can be drawn between Tables S4 and S5.
(E) Comparison of the fusion peptides of S370.7-bound LIV GPC (teal) with unbound LIV GPC (PDB: 8EJD; yellow).