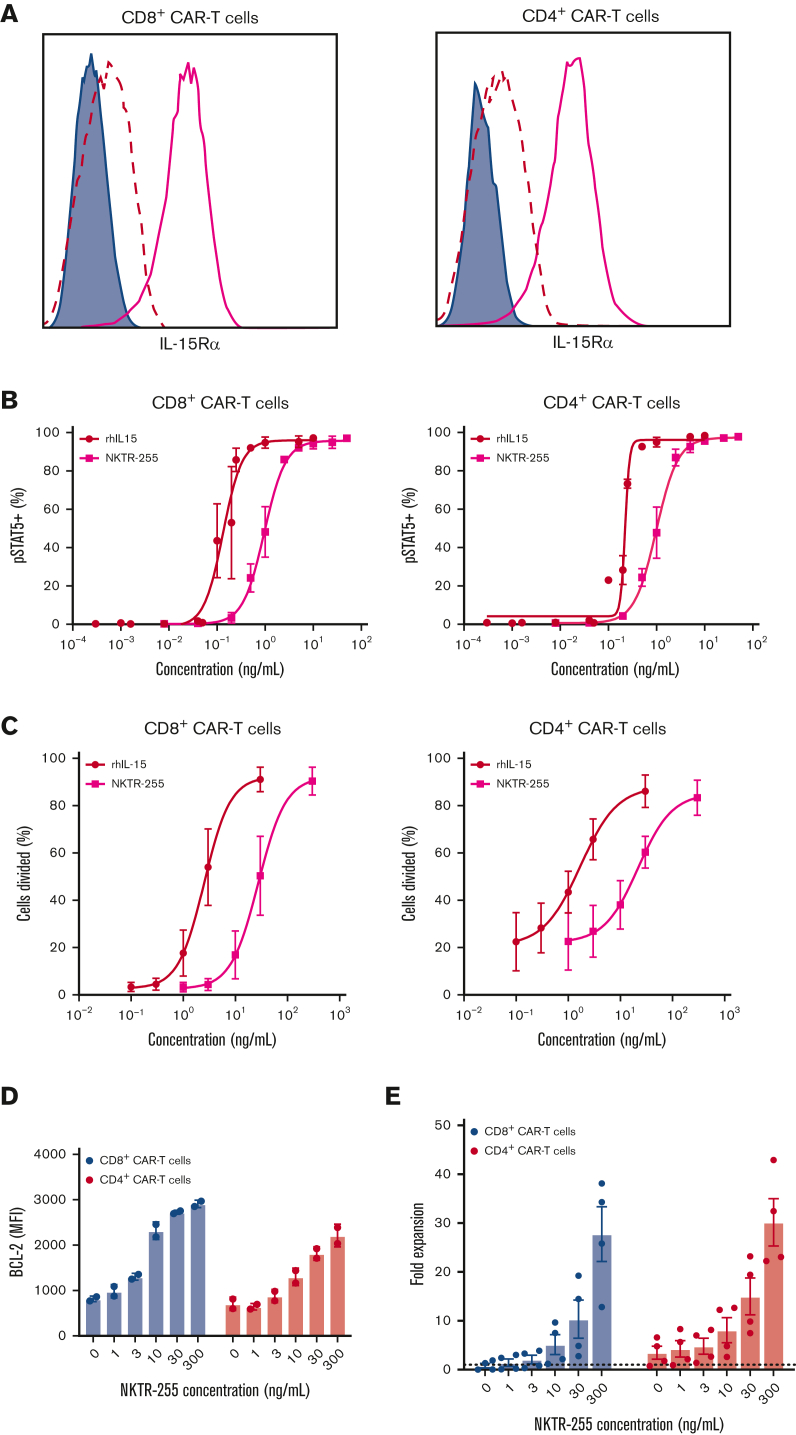
Figure 3.
Human CD19 CAR T cells exhibit a dose-dependent response to NKTR-255. Human CD19 CAR T cells were generated from healthy donors (n = 2-4) and assayed on days 14 to 16 after the start of manufacturing. (A) Representative surface expression of IL-15Rα (bold pink line) by flow cytometry on CD8+ (left) and CD4+ (right) CAR T cells. Filled histograms depict FMO and dashed lines represent isotype control. (B) CD8+ (left) and CD4+ (right) CAR T cells were incubated with the indicated concentrations of rhIL-15 or NKTR-255 for 20 minutes and phosphorylation of STAT5 was measured by flow cytometry. (C) CD8+ (left) and CD4+ (right) CAR T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and incubated with the indicated concentrations of rhIL-15 or NKTR-255 for 4 days. The percentage of divided cells was determined by CFSE dilution using flow cytometry. (D-E) CD8+ and CD4+ CAR T cells were incubated with the indicated concentrations of NKTR-255 for 4 days. (D) BCL-2 expression (mean fluorescence intensity) was measured using intracellular flow cytometry. (E) Fold expansion was determined by the fold change in absolute cell counts from days 0 to 4 by flow cytometry using counting beads. (B-E) Figures show the mean ± SEM. MFI, mean fluorescence intensity.