Significance
Ammonia-oxidizing archaea (AOA) are the major source of marine nitrous oxide (N2O); however, the cellular kinetics and pathways of archaeal N2O production remain unclear. We characterize N2O production kinetics of a model marine AOA species Nitrosopumilus maritimus at low ammonia concentrations and quantify the relative contributions of multiple N2O production pathways using dual 15N-18O isotope labeling. We provide direct evidence for the enzymatic regulation of N2O production by AOA. We found hydroxylamine oxidation contributes substantially to N2O production, which had not been previously recognized, and that nitrite reduction is not a significant source of N2O. These findings are important for the interpretation of pathways and regulation of N2O production in the ocean.
Keywords: nitrous oxide, ammonia-oxidizing archaea, dual isotope, marine N2O production pathways, kinetics
Abstract
The ocean is a net source of the greenhouse gas and ozone-depleting substance, nitrous oxide (N2O), to the atmosphere. Most of that N2O is produced as a trace side product during ammonia oxidation, primarily by ammonia-oxidizing archaea (AOA), which numerically dominate the ammonia-oxidizing community in most marine environments. The pathways to N2O production and their kinetics, however, are not completely understood. Here, we use 15N and 18O isotopes to determine the kinetics of N2O production and trace the source of nitrogen (N) and oxygen (O) atoms in N2O produced by a model marine AOA species, Nitrosopumilus maritimus. We find that during ammonia oxidation, the apparent half saturation constants of nitrite and N2O production are comparable, suggesting that both processes are enzymatically controlled and tightly coupled at low ammonia concentrations. The constituent atoms in N2O are derived from ammonia, nitrite, O2, and H2O via multiple pathways. Ammonia is the primary source of N atoms in N2O, but its contribution varies with ammonia to nitrite ratio. The ratio of 45N2O to 46N2O (i.e., single or double labeled N) varies with substrate ratio, leading to widely varying isotopic signatures in the N2O pool. O2 is the primary source for O atoms. In addition to the previously demonstrated hybrid formation pathway, we found a substantial contribution by hydroxylamine oxidation, while nitrite reduction is an insignificant source of N2O. Our study highlights the power of dual 15N-18O isotope labeling to disentangle N2O production pathways in microbes, with implications for interpretation of pathways and regulation of marine N2O sources.
Ammonia-oxidizing archaea (AOA) are ubiquitous and abundant members of the marine plankton; they are almost exclusively responsible for ammonia oxidation in the world ocean (1, 2). Globally, over 80% of marine nitrous oxide (N2O) is estimated to be produced as a side product of ammonia oxidation (3–5), indicating a dominant role of marine AOA in determining N2O distribution and its flux from ocean to atmosphere. Compared to their bacterial counterparts, ammonia-oxidizing bacteria (AOB), marine AOA lack the genes encoding the known bacterial machineries for N2O production (6) and exhibit lower N2O yield (7–9), implying distinct mechanisms of N2O production between AOA and AOB (10–12). The marine AOA demonstrate significantly higher affinity toward total ammonia (NH3 plus NH4+, hereafter referred to as NH4+) for ammonia oxidation during nitrite (NO2−) production than AOB (2), but the cellular kinetics of N2O production have not been explored. Hydroxylamine (NH2OH) and nitric oxide (NO) have been identified as key intermediates in AOA metabolism (13–15), implying multiple potential pathways for N2O production as both NH2OH and NO are likely precursors of N2O. However, the explicit pathways of archaeal N2O production are still incompletely known and remain controversial (10–12). The hybrid N2O formation pathway (in which the two N atoms in N2O are derived from different sources via the abiotic reaction between NH2OH and NO) has been experimentally demonstrated and is considered the dominant pathway for N2O production in AOA cultures and natural environments (Fig. 1A) (10–12). Other AOA pathways may include N2O production via NO2− reduction at low pH (16) and a novel NO dismutation pathway in marine AOA under anaerobic conditions (17). In contrast, no experimental evidence that AOA can directly convert NH2OH to N2O via NH2OH oxidation, either enzymatically or abiotically, has been reported. It is important to determine which pathways occur during archaeal ammonia oxidation and which are relevant in various environmental conditions because 1) the pathway and rate of N2O production might vary with NH4+ and NO2− availability, as both are involved in N2O formation; and 2) different sources of N and O used by AOA to produce N2O may impart distinct isotope signatures, which are used to deduce the sources of N2O in natural and man-made systems.
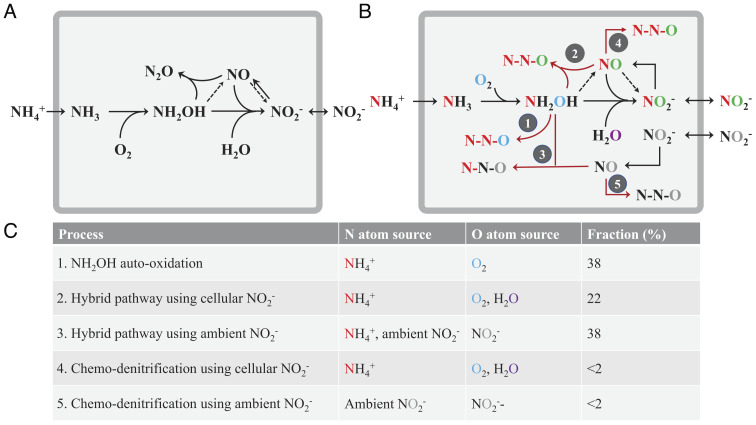
Fig. 1.
Summary of N2O production pathways during marine archaeal ammonia oxidation. (A) The hybrid pathway, which combines one N atom from NH2OH and one from nitrite (either ambient or newly produced inside the cell), has been observed previously (10–12). Black arrows represent known pathways for N atoms; dashed pathways represent hypothesized pathways involving NO. (B) Potential N2O pathways and the N and O atom sources identified in the current study. Five N2O pathways are identified: 1) NH2OH oxidation; 2) hybrid pathway a: NH2OH reaction with NO that was sourced from the reduction of newly produced NO2−; 3) hybrid pathway b: NH2OH reaction with NO that was sourced from ambient NO2−; 4) reduction of newly produced NO2−; and 5) reduction of ambient NO2−. Color of the N and O atoms depicts the sources: red and black denote N atoms from NH4+ and ambient NO2−, respectively; blue, purple, and gray denote O atoms from O2, H2O, and ambient NO2−, respectively; green represents O atoms of newly produced NO2−, which is a mixture of H2O and O2. The gray square denotes the membrane and periplasmic space of the AOA cell. The black arrows represent the ammonia oxidation pathway, the red arrows show the potential N2O production processes, and the dashed arrows indicate potential pathways that are unresolved in our study. (C) Summary of the N, O atom sources of N2O and the fractional contribution of each pathway during marine archaeal ammonia oxidation with initial NH4+: NO2− ratio of 1:1.
Elucidation of all potential sources of N2O, however, remains a challenging task. To date, most investigations on AOA N2O production pathways have focused on the source of the nitrogen (N) atoms (13, 16–18). However, using N isotopes alone is insufficient to distinguish multiple N2O production pathways that occur simultaneously and to quantitatively estimate the relative contribution of each pathway. For instance, the 15N-NH4+ isotope labeling approach cannot completely distinguish N2O production through NH2OH oxidation, hybrid formation, or NO2− reduction, because all these precursors of N2O could ultimately be sourced from NH4+ during ammonia oxidation. The 18O-labeling approach provides an independent avenue to identify the source of N2O by tracking the oxygen (O) atom in N2O. A few studies have used 18O-H2O to show that the O atom in N2O can be partly derived from H2O in lab culture and field studies (9, 19, 20). However, the alternative sources of the O atom in N2O are still unknown. Importantly, because the O atom source differs among the various N-containing precursors, i.e., NH2OH, NO2−, and NO, dual 15N and 18O isotope labeling is a powerful method to disentangle the complex and interconnected N2O production pathways in AOA. In this study, using a model marine AOA species Nitrosopumilus maritimus strain SCM1 (hereafter refer to as SCM1), we conducted a comprehensive set of dual-isotope labeling incubation experiments to systematically investigate N2O production kinetics and the associated pathways during archaeal ammonia oxidation under various substrate conditions (SI Appendix, Table S1).
Results and Discussion
Kinetics of N2O Production during Archaeal Ammonia Oxidation.
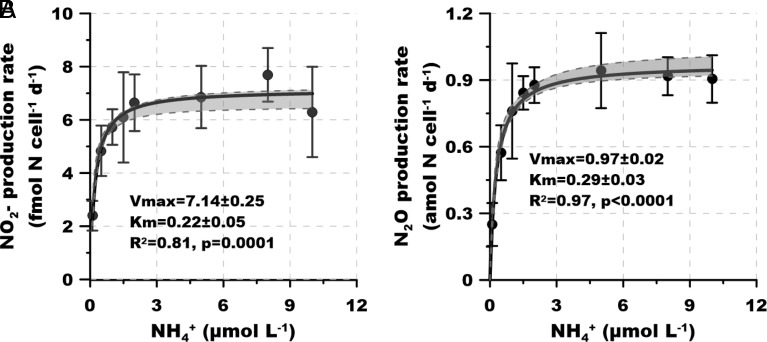
Marine AOA have a remarkably high affinity for NH4+, which is consistent with their dominant role in ammonia oxidation to NO2− in oligotrophic marine environments (2). However, the effect of NH4+ concentrations on N2O production by AOA remains unknown. If N2O is primarily generated via abiotic hybrid reactions between intermediates of archaeal ammonia oxidation, N2O production by AOA may not follow normal enzyme kinetics. We found that both NO2− and N2O production rates varied with NH4+ concentration (Experiment 1) and both followed Michaelis-Menten-type kinetics (Fig. 2 A and B). The apparent half saturation constants (Km(app)) for NO2− production (220 ± 50 nmol L−1 NH4+) were comparable to those that were previously determined for ammonia (132 nmol L−1 NH4+) and oxygen uptake (133 nmol L−1 NH4+), suggesting all essential enzymatic steps for ammonia oxidation and respiration are highly efficient and tightly coupled at low ammonia concentrations in marine AOA. Likewise, although the maximum rate (Vmax) for N2O production (0.97 ± 0.02 amol N cell−1 d−1) was more than three orders of magnitude lower than that for NO2− production (7.14 ± 0.25 fmol N cell−1 d−1), Km(app) values for the two rates were comparably low. The comparable Km(app) values for ammonia during NO2− and N2O production imply that both are controlled by enzyme activity in SCM1 at low NH4+ concentrations. This implies that enzyme activity provides the key intermediates NH2OH and NO for both NO2− and N2O production, i.e., there is no separate completely abiotic reaction that is responsible for N2O production. The Km(app) for N2O production is four orders of magnitude lower than the Km reported for NO production by SCM1 measured during ammonia oxidation (measured at >2 mol L−1 NH4+) (14). Therefore, the supply of NO is unlikely to be a limiting factor in determining the kinetics of N2O production, implying a critical role for NH2OH in determining the observed kinetics.
Fig. 2.
Experiment 1. Kinetics of ammonia oxidation and N2O production by SCM1. (A) Michaelis-Menten-type plot of substrate-dependent rate of ammonia oxidation to NO2− (normalized to per cell per day). (B) Michaelis-Menten plot of substrate-dependent rate of N2O production. Error bars represent SD from triplicate samples. The black lines and gray shadows show the Michaelis-Menten type regressions and the 95% CIs, respectively.
NH2OH is enzymatically produced by ammonia monooxygenase and rapidly converted to NO2− (15). The tight coupling of its production and consumption in AOA species at low NH4+ concentrations results in its limiting conversion to N2O as a side product. However, a small fraction of NH2OH can escape from being oxidized by the enzymatic reaction, providing the key precursor for N2O production. For example, only 0.46% of NH2OH was released during ammonia oxidation by the AOA Nitrososphaera gargensis even at very high NH4+ concentrations [2 mmol L−1 (21)], which is one to two orders of magnitude lower than the reported NO accumulation ratio during ammonia oxidation by SCM1 (14). The similarly high affinities (low Km) for NO2− and N2O production further suggest that both kinetics were determined by the NH2OH supply; if the conversion of NH2OH to NO2− was the rate-limiting step, the production of N2O should increase continuously with the accumulation of NH2OH. These results suggest that the SCM1-like marine AOA effectively use the trace level NH4+ in the vast N-depleted ocean for both NO2− and N2O generation, providing direct evidence for the capability of marine AOA to dominate N2O production in the ocean and its subsequent release to the atmosphere.
Impact of NH4+: NO2− Ratio on Pathways of N2O Production.
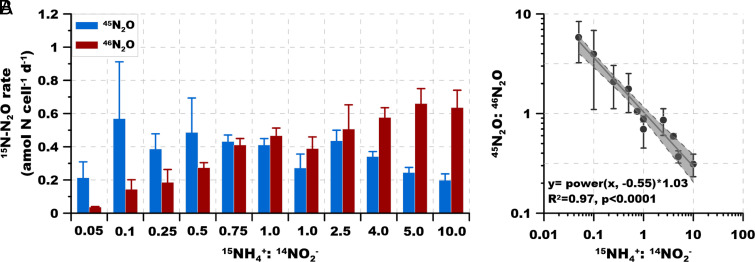
Both NH4+ and NO2− are involved in N2O production by AOA (9, 18), but the rates and pathways might vary depending on relative substrate availability. Therefore, we investigated N2O production under a wide range of 15NH4+: 14NO2− ratios (from 0.05 to 10) (Experiments 1 and 2). The ratio of single labeled N2O to double labeled N2O (45N2O: 46N2O) decreased from 5.82 ± 2.58 to 0.37 ± 0.05 as the 15NH4+: 14NO2− ratio increased from 0.05 to 10 (Fig. 3A and SI Appendix, Fig. S1). This dependence on substrate ratio indicates that 45N2O: 46N2O ratio is not a constant but is highly variable and implies that more than one pathway contributes to N2O production in AOA. The strong and significant correlation (R2 = 0.97, P < 0.0001) between the 45N2O: 46N2O ratio and substrate 15NH4+: 14NO2− ratio implies that the relative contributions of different pathways to N2O production and the source of the N atoms in N2O should vary with NH4+: NO2− ratio in the environment (Fig. 3B).
Fig. 3.
Experiment 2. 15N-N2O production and isotope composition under different 15NH4+ 14NO2− ratios. (A) 45N2O (single labeled) and 46N2O (double labeled) production rate under different 15NH4+ and 14NO2− concentrations (normalized to per cell per day). All of the 45N2O represents hybrid formation. (B) Regression between 45N2O: 46N2O production rate against 15NH4+ 14NO2− concentration ratio. Error bars represent SD from triplicate samples.
NH4+ and NO2− are highly dynamic nitrogen cycle components that rarely accumulate in the global ocean. However, NH4+ and NO2− can accumulate in specific regions (e.g., oxygen minimum zones and eutrophic waters) and depths (e.g., NH4+ maximum, primary NO2− maximum), leading to substantial variation of NH4+: NO2− ratio in these biogeochemically active marine environments (22). Our data indicate that N2O can be produced via distinct pathways by marine AOA and sourced from different N atoms in waters with different ratios of NH4+: NO2−, even though the main source process is always archaeal ammonia oxidation. For example, at the primary NO2− maximum where NO2− accumulates, the low NH4+: NO2− ratio might lead to the higher contribution of NO2− to N2O production via the hybrid pathway. In contrast, at the NH4+ maximum and certain hotspots of NH4+ supply such as zooplankton excretion or decay of phytoplankton blooms (23), NH4+ would dominate N2O formation under the elevated NH4+: NO2− ratio. These findings provide new insights in interpreting the natural abundance isotope signature of N2O in the water column of the global oligotrophic oceans, where a subsurface dual-isotope minimum is consistently observed and has been widely interpreted as resulting from N2O production via nitrifier-denitrification (24–27). These new data would suggest, however, that low NH4+: NO2− ratio (i.e., relatively higher NO2− concentration) would also lead to the observed dual-isotope minimum by the incorporation of more isotopically depleted NO2− by the hybrid pathway during the ammonia oxidation process.
Our results are also important for the interpretation of isotope labeling patterns observed in isotope tracer experiments in the ocean, where production of 45N2O has been generally attributed to the hybrid N2O formation pathway (one N from NH4+, one N from NO2−). We suggest that 46N2O could also be partially hybrid, from the combination of 15NH4+ and newly produced 15NO2−. The contribution of the hybrid pathway would be underestimated by ignoring 46N2O production. However, our results cannot fully explain the high fraction of 45N2O production (i.e., >70%) in the ocean (24, 28–30), nor the finding that the %46N2O is insensitive to short-term experimental NO2− enrichment (31) (comparison to field observations; SI Appendix, Text 1). Nevertheless, the variable N2O atom composition by SCM1 under different NH4+: NO2− ratio provides new insights into marine N2O pathways and interpretation of its isotope composition. These pathways and the relative contributions of N and O from multiple sources are explored in experiments described below.
Contribwutions of NH4+ and NO2− to N2O Production.
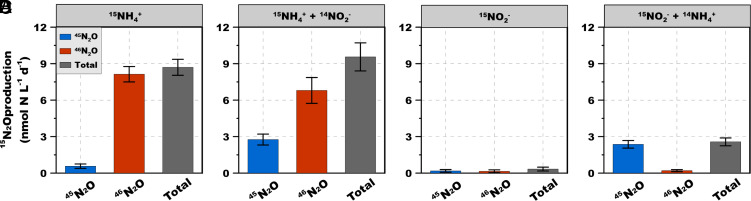
The potential pathways and relative contributions of the two substrates, NH4+ and NO2−, via various pathways of N2O formation in AOA were explored using multiple tracer combinations (Experiment 3). NH4+ and NO2− concentrations were controlled by adding the substrates to cells that had first been washed and resuspended in substrate-free fresh medium. When 15NH4+ was added without 14NO2−, double labeled 46N2O was the main product (93.4 ± 10.1% of the total labeled N2O production rate) (Fig. 4A). 15NH4+ was the only N source in the experiment, so the small production of 45N2O (6.6% of the total labeled N2O production) can be attributed to trace amounts of intracellular 14NH4+ and/ or 14NO2−, or to carry over from the inoculum. When equimolar amounts of 15NH4+ and 14NO2− were provided, the fractional contribution of 45N2O increased to 28.9%, indicating that ambient NO2− is involved in 45N2O production, although the labeled N2O pool was still primarily 46N2O. This hybrid N2O formation indicates involvement of ambient NO2− in N2O production, but the process varies among AOA strains (18) and with substrate ratio (Fig. 3B). Newly produced (presumably intracellular, or at least in the pseudo-periplasmic space) and ambient NO2− might both be involved in N2O hybrid formation (pathways 2, 3 in Fig. 1B). Total labeled N2O (combined 45N2O and 46N2O) production rate in the 15NH4+ tracer incubation (8.7 ± 0.7 nmol N L−1 d−1, Fig. 4A) was comparable to the rate in the 15NH4+ + 14NO2− incubation (9.6 ± 1.5 nmol N L−1 d−1, Fig. 4B), indicating no discernible difference between the effects of ambient and newly produced NO2− on N2O production rate by SCM1.
Fig. 4.
Experiment 3. 15N-N2O production during 15NH4+ and 15NO2− labeling incubations using viable cells. (A–D) l5N labeled N2O production rate from 15NH4+, 15NH4+ + 14NO2−, 15NO2−, and 15NO2− + 14NH4+ labeling incubations, respectively. Error bars represent SD from triplicate incubations. Total N2O refers to total labeled N2O (45N2O + 46N2O). Approximately, 6 nmol N L−1 d−1 44N2O must have been produced in the 15NO2− + 14NH4+ incubation (D), but the amount of 44N2O could not be determined in these experiments due to lack of sensitivity in small volume incubations. 44N2O would not have been present in the other three experiments because in A all the NH4+ was labeled; in B we have shown that N2O cannot be formed from NO2− alone, and C all the NO2− was labeled.
15N-labeled N2O production from 15NO2− tracer was negligible (0.3 ± 0.2 nmol N L−1 d−1) in the absence of NH4+ (Fig. 4C). By comparison, when 14NH4+ was added with 15NO2−, the 15N-labeled N2O production rate increased significantly to 2.6 ± 0.3 nmol N L−1 d−1 (P < 0.001), which is strong evidence for a hybrid N2O formation mechanism that involves both intermediates from ammonia oxidation and NO2− reduction (Fig. 4D). It is not surprising that N2O production rate decreased greatly in the absence of NH4+, as NH4+ is the substrate for energy generation and the source of the key N2O precursor NH2OH. Thus, N2O can be produced from NH4+ alone in the absence of NO2−, but N2O cannot be produced from NO2− alone. When NH4+ is present, however, NO2− contributes to N2O formation via a hybrid pathway. In contrast to the 15NH4+ tracer experiment, 45N2O dominated the 15N-N2O pool in the 15NO2− + 14NH4+ incubation (92.1 ± 16.8%). The small fraction (7.9 ± 3.4%) of 46N2O detected in this treatment indicated a minor contribution of the chemo-denitrification-like pathway (i.e., both N atoms from NO2−, pathways 4, 5 in Fig. 1B) to N2O production (Fig. 4D) (13). However, the 46N2O production rate from 15NO2− measured here was two orders of magnitude lower than rates measured when external oxygen was exhausted (5 to 22 nmol L−1 h−1) (17), indicating production of N2O in AOA by the proposed NO2− reduction-NO dismutation pathway is restricted to anoxic conditions.
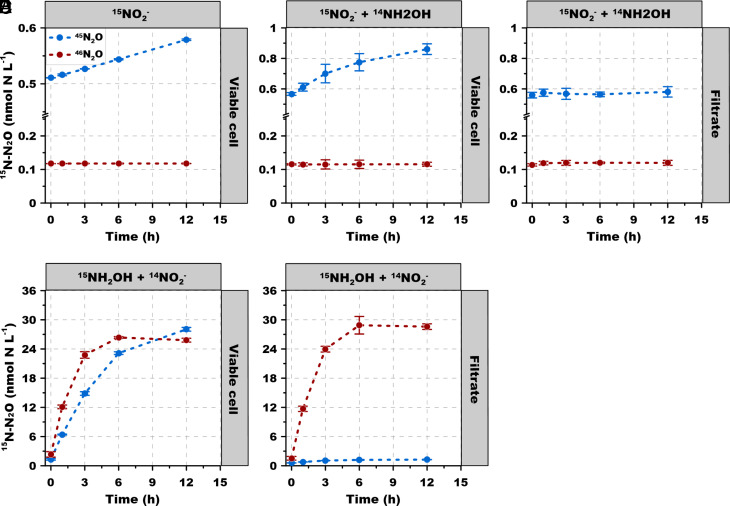
The Role of NH2OH as Key Precursor for N2O Production.
Although NH2OH is not an important N source in the marine environment (its reactivity guarantees a very low ambient concentration) (32), it is a critical intracellular intermediate in archaeal ammonia oxidation and N2O production. Experiments using 15NH2OH and 15NO2− + 14NH2OH were used to explore the pathways by which NH2OH participates in N2O production during ammonia oxidation (Experiment 4). Notably, here we used 1 μmol L−1 of NH2OH concentration, which was two orders of magnitude lower than previous studies that explored N2O pathways by AOA (i.e., 200 μmol L−1) (13), thus closer to environmental concentrations but also ensuring the sensitivity of the assays to probe the potential mechanisms. Moreover, no discernible difference was observed for NO2− production with or without NH2OH amendment, suggesting no detectable inhibition of 1 μmol L−1 of NH2OH on archaeal ammonia oxidation (P > 0.05) (SI Appendix, Fig. S2).
45N2O increased slowly but continuously over 12 h from tracer 15NO2− in viable cells, while no discernible 46N2O accumulation was detected (Fig. 5A). 14NH2OH added with 15NO2− stimulated 45N2O production from 15NO2− in viable cells, indicating 14NH2OH was directly involved in the reaction with 15NO2− to produce hybrid 45N2O (Fig. 5B). In contrast, 45N2O production nearly stopped after removing the cells by filtration. The fact that the viable cells produced more 45N2O from the 15NO2− tracer than the filtrate indicates that cellular metabolism facilitated N2O production from the hybrid pathway, and that abiotic formation rate of N2O by NO2− and NH2OH was low (Fig. 5C).
Fig. 5.
Experiment 4. 15N-N2O production from NH2OH. l5N labeled N2O production rate from (A) viable cells with 15NO2− tracer (10 μmol L−1); (B) viable cells with 15NO2− (10 μmol L−1) + 14NH2OH (1 μmol L−1); (C) filtrate with 15NO2− (10 μmol L−1) + 14NH2OH (1 μmol L−1); (D) viable cells with 15NH2OH (1 μmol L−1) + 14NO2− (50 μmol L−1); (E) filtrate with 15NH2OH (1 μmol L−1) + 14NO2− (50 μmol L−1). Error bars represent SD from triplicate samples.
Much higher labeled N2O production rates occurred in incubations supplemented with 15NH2OH (Fig. 5 D and E) than in incubations supplemented with either 15NO2− alone or 15NO2− + 14NH2OH, despite the concentration of 15NO2− (10 μmol L−1) being tenfold higher than 15NH2OH (1 μmol L−1), demonstrating active involvement of NH2OH in N2O production. In the presence of viable cells, both 45N2O and 46N2O were produced (Fig. 5D), while for the filtrate, only 46N2O production was observed (45N2O accounted for <5% of the total labeled N2O production) (Fig. 5E). The comparable 46N2O production between viable cell and filtrate groups indicated that 46N2O was mainly produced via abiotic NH2OH oxidation, and NO2− is not involved in that reaction. In contrast, viable cells are needed for the production of 45N2O from 15NH2OH and 14NO2−, suggesting the hybrid reaction may require enzymatic activity to produce NO from NO2−. Therefore, it appears that the ambient NO2− can enter the periplasmic space of the cell for the production of NO that is most likely catalyzed by a putative periplasmic copper-containing nitrite reductase (10, 11). These results indicate the central role of NH2OH as a precursor of N2O (pathways 1, 2, 3, and 4 in Fig. 1B). Moreover, the co-production of 45N2O and 46N2O from 15NH2OH shows that hybrid N2O formation and oxidation of NH2OH both contributed to N2O production in viable cells. Note that the high affinity for NH4+ and the typical dependence of N2O production rate on NH4+ concentration (Experiment 1) implicate enzymatic control of N2O production, even though the last step in both pathways (hybrid and NH2OH oxidation) is abiotic.
Taking all the results with 15N-labeled substrates together, these findings demonstrate that N derived from both NH4+ and NO2− is involved in N2O production by AOA, but that NH4+ is the major N source. Under the initial NH4+: NO2− ratio of 1:1, NH4+ accounts for ~85% of N atoms to N2O. Paired analysis of 45N2O: 46N2O further implied that an additional N2O formation pathway solely sourced from NH4+ was required to explain the dominance of NH4+ as the source of N atoms in N2O. Thus, apart from the hybrid formation pathway, NH2OH oxidation might be an important and previously overlooked pathway that contributes to archaeal N2O production. However, it is difficult to discriminate all the associated pathways and to quantify the contributions of each process using N isotopes alone, because all N atoms in N2O precursors could be directly or indirectly sourced from NH4+.
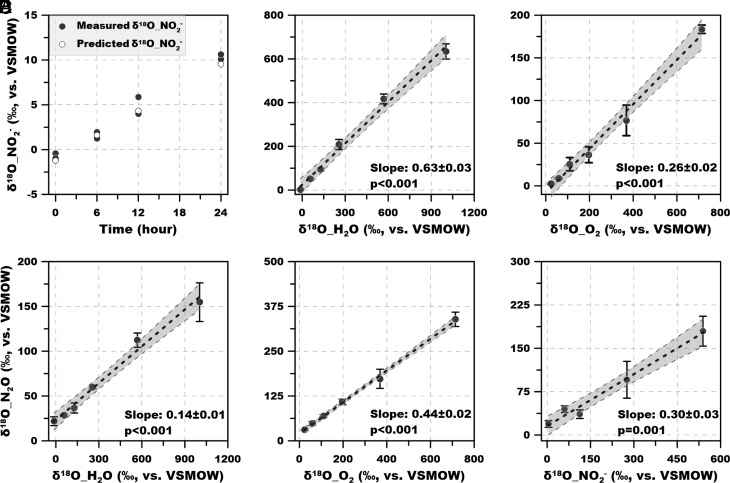
Tracing the O in N2O: Contributions of H2O, O2, and NO2− as the Source of O in N2O.
18O leaves footprints in N2O and NO2− that are independent of 15N and can thus provide further information on the pathways to N2O. We developed a comprehensive set of 18O-labeling experiments to determine the source of O atoms in both NO2− and N2O, and to quantify potential N2O production pathways from H2O, NO2−, and O2 (Experiment 5). The isotopic enrichment of δ18O-NO2− showed an approximately linear increase over time in the 24 h abiotic O atom exchange experiment, i.e., from 18O-H2O in the absence of viable cells (Fig. 6A). The measured values of δ18O-NO2− agreed well with the amount of δ18O-NO2− predicted using an exchange rate constant of 0.117 (33) under the experimental conditions (pH: 7.8; temperature: 30°C).
Fig. 6.
Experiment 5. δ18O of the produced NO2− and N2O during the 24 h 18O-labeling incubations. (A) Change of δ18O-NO2− due to abiotic O atom exchange. (B and C) δ18O of the produced NO2− in 18O-H2O and 18O-O2 labeling experiments. (D–F) δ18O of the produced N2O in 18O-H2O, 18O-O2, and 18O-NO2− labeling experiments, respectively. Dashed lines denote the best linear regression, and gray shadow represents 95% CIs. Error bars represent SD from triplicate samples.
This correction for abiotic O atom exchange was applied to determine the δ18O of the produced NO2− in incubations with viable cells. O atoms from both H2O and O2 were incorporated into NO2− during archaeal ammonia oxidation (and the amount of δ18O-NO2− was proportional to the amount of labeled substrate in both 18O-H2O and 18O-O2 labeling incubations) (Fig. 6 B and C). The slope of δ18O-NO2− vs. δ18O-H2O (63 ± 3%) was significantly greater than the slope of δ18O-NO2− vs. δ18O-O2 (26 ± 2%) (P < 0.001). The significantly higher contribution of H2O than O2 to the O atoms in NO2− is consistent with the hypothesis that NH2OH and NO act as co-substrates to produce two molecules of NO2−. Then one NO2− molecule is reduced back to NO and another O atom from H2O is incorporated into NO2− (13). Alternatively, an intracellular O atom exchange could occur during NO2− production by AOA (SI Appendix, Text 2).
δ18O of the produced N2O increased with increasing δ18O-H2O, δ18O-O2 and δ18O-NO2−, indicating that O atoms from all three potential sources were incorporated into N2O (Fig. 6 D–F) with different contributions. Interestingly, in contrast to the O atom source structure in NO2−, O2 contributed the largest fraction of O atoms to N2O (44 ± 2%), followed by NO2− (30 ± 3%) and H2O (14 ± 1%). Because the O atoms in NH2OH are sourced from O2 and no further exchange occurs between NH2OH and H2O (34), the fact that O2 (via NH2OH) contributed most to O atoms in N2O supports our finding of a substantial role for NH2OH oxidation in producing N2O (pathway 1 in Fig. 1B). The incorporation of O atoms from NO2− and H2O into N2O indicated internally produced and externally added (ambient) NO2− can both be involved in N2O production. In the absence of known nitric oxide reductase catalyzing NO reduction to N2O through nitrifier-denitrification, potential N2O pathways associated with NO2− in marine AOA include abiotic NO2− reduction (chemo-denitrification-like) and hybrid formation. However, our 15NO2− labeling incubations showed that NO2− was involved in N2O production only in the presence of NH4+, and NO2− alone did not contribute substantially to N2O production (Fig. 4 C and D). Therefore, hybrid formation is the dominant pathway by which NO2− contributes to N2O production. Moreover, the incorporation of O atoms from H2O and ambient NO2− further revealed that during hybrid formation, O atoms in NO2− or NO, rather than NH2OH, were retained in the N2O molecule. If the O atom was sourced from NH2OH during the hybrid process, all the O atoms should be contributed by O2. The incorporation of the O atom from ambient NO2− into N2O also shows that at least some NO was produced via NO2− reduction, as previously hypothesized (11, 14).
Quantifying Multiple N2O Sources during Archaeal Ammonia Oxidation.
The dual-isotope labeling method enabled us to fully resolve multiple N2O production pathways in AOA. Combining results from 15N and 18O-labeling incubations (Experiments 3 to 5), we can quantitatively estimate the fractional contribution of the five potential N2O production pathways (Fig. 1B). NO2− reduction (pathways 4 and 5) is an insignificant N2O source under aerobic growth conditions when both NH4+ and NO2− are present in equimolar amounts (Fig. 4). NH2OH is revealed as the main contributor to N2O production (Fig. 5) via both hybrid pathway and NH2OH oxidation. In NH2OH oxidation (pathway 1), 100% of O atoms in N2O were sourced from O2, while in hybrid formation (pathways 2 and 3), the O atom was derived from NO2− via reduction to NO. The NH2OH involved in hybrid N2O formation contributed an N atom but not the O atom. There were two sources of NO2−: the original ambient NO2− (100% of O atoms were sourced from ambient NO2−) and the NO2− newly produced from ammonia oxidation (63% of O atoms from H2O and 26% from O2) (Fig. 6). Under these conditions, we calculated the following contributions to O atoms in N2O: 38.2% from NH2OH oxidation (pathway 1), 59.8% from the hybrid source (22.2% by pathway 2 and 37.6% by pathway 3), and 2.1% from NO2− reduction (pathways 4 and 5) (Fig. 1C). This combination best fits the observed results that 14% of O atoms in N2O were from H2O and 44% of O atoms were from O2 under our experimental conditions. Although the fractional contribution of the pathways might vary with various substrate concentrations (i.e., different NH4+: NO2− ratios), the comprehensive 15N-18O dual-isotope labeling technique developed here provides a novel avenue to disentangle and quantify the relative contribution of multiple pathways to N2O production in both lab and field studies.
Archaeal ammonia oxidation is the primary source of marine N2O, yet the mechanistic understanding of archaeal N2O production remains elusive. We present the first study determining the kinetics of archaeal N2O production and provide strong evidence of the capability of marine AOA in producing N2O at trace levels of NH4+, supporting their dominant role in contributing to N2O production in the ocean. We further show a direct control of NH4+ and NO2− concentrations on the sources of N2O, providing new insights into understanding the varying isotope composition of N2O in the ocean. The increased incorporation of N and O atoms from NO2− into N2O by marine AOA at low NH4+: NO2− ratios suggests a new mechanism for interpreting the ubiquitous N2O isotope minimum without the need to invoke nitrifier-denitrification by AOB, which are rarely detected in the oligotrophic open ocean. Our comprehensive dual 15N-18O-labeling techniques identify a substantial contribution of NH2OH oxidation to archaeal N2O production that was previously not recognized. These explicit descriptions of the N2O production pathways and kinetics in AOA should improve our understanding of marine N2O production, and the multiple N and O atom sources of N2O identified here should inform biogeochemical models that aim to resolve the marine nitrogen cycle and constrain the air-sea N2O flux. Moreover, our dual-isotope labeling technique could be applied in combination with manipulative experiments, such as temperature, pH, and dissolved oxygen (DO), to explore rates and pathways of archaeal N2O production in response to ocean warming, acidification and deoxygenation.
Materials and Methods
SCM1 Cultivation and Isotope Labeling Incubation.
N. maritimus strain SCM1 was cultured in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffered Synthetic Crenarchaeota Medium (SCM) (pH: ~7.8) at 30 °C in the dark following Qin et al. (2014) (35). Six sets of incubations were carried out (SI Appendix, Table S1). The SCM1 cells were grown and maintained in 2-L bottles containing 600 mL SCM for the preservative test and experiments 3 to 5 and were grown in 100-mL bottles containing 40 mL SCM for experiments 1 to 2. The medium contains FeNaEDTA and other trace metals including, Cu, Ni, Zn, and Co. The approximate concentrations of the trace metals can be found in Amin et al. (2013) (36). The medium was supplemented with NH4Cl at different initial concentrations for each of the experiments: Initial concentrations were 1,000 μmol L−1 in the preservative test experiment, 500 μmol L−1 in experiments 3 and 5, 200 μmol L−1in experiment 1 and 2, and 100 μmol L−1 in experiments 4. Growth was monitored both by measuring NO2− concentration and NH4+ consumption and by performing cell counts using flow cytometry (Accuri C6, BD Biosciences). After determining the best strategy for terminating the incubation to preserve the concentration and isotopic content of analytes (Preservative Test), the five experimental incubations were carried out to 1) test the kinetic response of N2O production during SCM1 ammonia oxidation; 2) examine the impact of NH4+: NO2− substrate ratio on the pathways and composition of N atoms in N2O; 3) track N atom sources through 15N labeling experiments; 4) test the contribution of ambient NH2OH to N2O production; and 5) track O atom sources through multiple 18O-labeling experiments. All labeling incubation experiments were performed using mid- to late-exponential phase cultures.
Preservative test.
A total of ~1.0 L of culture was collected and aliquoted into two groups in the mid-exponential phase: 1) viable cells and 2) killed control (autoclaved at 120°C for 30 min and cooled overnight). In each group, each 15N tracer (15NH4+ and 15NO2−, 99% 15N, Cambridge, United States) was added to separate bottles to a concentration of 50 μmol L−1. For the viable cells, one additional treatment (100 μmol L−1 of 15NO3−, 98% 15N, Cambridge, United States) was performed. After tracer addition, 10 mL of sample was dispensed into triplicate 20-mL serum bottles and sealed with 20-mm butyl stoppers and aluminum crimp seals (Wheaton, United States). Two preservatives (20 μL of saturated HgCl2 and 500 μL of 10 mol L−1 of NaOH) were used to compare the effect of preservatives on terminating biological activity and archaeal N2O production. For the viable cells, the incubations were performed at 30°C in the dark and terminated at 0 and 24 h. For the autoclaved samples, the preservatives were added only at 0 h. All incubations were performed in triplicate. Our results showed that HgCl2 induces artifacts of N2O production from pathways involving NO2−. Such artifacts are negligible when using NaOH as a preservative (SI Appendix, Fig. S3 and Text 3). Thus, NaOH was chosen as the preservative for all further experiments.
Experiment 1: Kinetic test.
When all amended NH4+ (200 μmol L−1) was completely consumed (i.e., below the substrate threshold of SCM1, ~10 nmol L−1 NH4+), 1% inoculum was transferred into NH4+ free fresh medium supplemented with labeled tracers. The initial cell abundance was around 1.39 × 105 cells ml−1, which is comparable to AOA cell abundance in the ocean, and initial carry over 14NO2− concentration was ~2 μmol L−1. A total of eight 15NH4+ concentrations (0.1, 0.5, 1, 1.5, 2, 5, 8, 10 μmol L−1) were used for the kinetic test. Immediately after tracer amendment, 50 mL aliquots of sample were dispensed into 60-mL serum bottles and sealed with 20-mm butyl stoppers and aluminum crimp seals (Wheaton, United States). The incubation was performed at 30°C in the dark. Depending on the initial 15NH4+ concentration, the incubation was terminated at 0 h, ~2 h (0.1 μmol L−1), ~6 h (0.5 μmol L−1), and ~12 h (>0.5 μmol L−1) by adding 2.5 mL of 10 mol L−1 of NaOH. A third time point (~24 h) was also applied for all treatments. However, the third time point was only used for rate calculation in the high NH4+ treatments (>1.5 μmol L−1) because the substrate was nearly completely consumed or exhausted before 24 h in those low substrate treatments. The incubations were carried out in triplicates at each time point.
Experiment 2: Substrate ratio experiment.
When 100 μmol L−1 of NH4+ was consumed, 1% inoculum was transferred into NH4+ free medium. The initial cell abundance was around 6.2 × 104 cells mL−1. Three NH4+: NO2− ratios were achieved by adding different amounts of 15NH4+ and 14NO2− (10 and 1 μmol L−1 of NH4+ and NO2−, 5 and 5 μmol L−1 of NH4+ and NO2−, and 1.5 and 15 μmol L−1 of NH4+ and NO2−, respectively). Immediately after tracer amendment, 40 mL aliquots of sample were dispensed into 60-mL serum bottles and sealed with 20-mm butyl stoppers and aluminum crimp seals (Wheaton, United States). The incubation was performed at 30°C in the dark and terminated by adding 2 mL of 10 mol L−1 of NaOH at 0, 6 and 24 h with triplicates at each time point.
Experiment 3: Source of N atoms in N2O.
A total of ~4 L of culture was harvested by gentle filtration onto two 0.2 μm pore size Sterivex filters (Millipore). Immediately after the filtration, the filters were flushed using 2-L fresh substrate-free medium to collect the cells and to remove the high background NH4+ (~210 μmol L−1) and NO2− (~330 μmol L−1). The cell densities before (4 L original culture) and after the filtration (resuspended in 2 L medium) were 1.15 × 107 and 2.10 × 107 cells mL−1, respectively, demonstrating a good recovery efficiency (~60%) of the pre-concentration process. The measured ammonia oxidation rate of the washed cells (~16 μmol N L−1 d−1) was lower than the rate of unwashed cells measured on the same day (~84 μmol N L−1 d−1). This reduced oxidation rate indicates that the manipulation process caused physiological stress on the SCM1 cells and resulted in decreased cellular activity. Nevertheless, the activity of the washed cells was high enough to allow precise measurement of rates of ammonia oxidation and N2O production in the experiments. The recovered cells were aliquoted into eight acid washed 250-mL PC bottles (Nalgene) and for four groups of tracers (15NH4+, 15NH4+ + 14NO2−, 15NO2−, 15NO2− + 14NH4+). Immediately after tracer amendment, 10 mL aliquots of sample were dispensed into 20-mL serum bottles and sealed with 20-mm butyl stopper and aluminum crimp seals (Wheaton, United States). The incubation was performed at 30°C in the dark and terminated by adding 500 μL of 10 mol L−1 of NaOH at 0 and 24 h with triplicates at each time point.
Experiment 4: Role of NH2OH in N2O production.
Around 1 L of culture was aliquoted into five groups when 50 μmol L−1 of NH4+ had been oxidized: 1) viable cells amended with 15NO2− (10 μmol L−1); 2) viable cells amended with 15NO2− (10 μmol L−1) + 14NH2OH (1 μmol L−1); 3) filtrate (through 0.2 μm PES filter) amended with 15NO2− (10 μmol L−1) + 14NH2OH (1 μmol L−1); 4) viable cells amended with 15NH2OH (1 μmol L−1); and 5) filtrate (through 0.2 μm PES filter) amended with 15NH2OH (1 μmol L−1). After tracer amendment, 10 mL aliquots of sample were dispensed into 20-mL serum bottles and sealed with 20-mm butyl stoppers and aluminum crimp seals (Wheaton, United States). Time-course incubation (0, 1, 3, 6, 12 h) was carried out for all the groups, and the incubation was performed at 30°C in the dark with triplicates and terminated by adding 500 μL of 10 mol L−1 of NaOH at each time point.
Experiment 5: Source of O atoms in N2O.
A total of ~7.2 L of culture was harvested by gentle filtration onto two 0.2-μm pore size Sterivex filters (Millipore). Immediately after the filtration, the filters were back flushed using 2.5 L fresh substrate-free medium to collect the cells and to remove the high background NH4+ and NO2−. Three groups of tracers (H218O, 18O2 and N18O2−) were used to track the source of the O atom. For the 18O-H2O labeling experiment, a range of δ18O-H2O tracer amendments (−13 to 1003‰) were made by adding 0.2 mL of 18O-H2O stocks with different 18O enrichment into the samples (18O-H2O stocks were made by mixing the H218O (99% 18O, Sigma-Aldrich, United States) with distilled deionized H2O). Similarly, six levels of δ18O-O2 (24 to 714‰) were made by adding 0.2 mL of 18O-O2 stocks with different 18O enrichment (18O-O2 stocks were made by mixing the 18O2 (99% 18O, Sigma-Aldrich, United States) with He) into the samples. For the 18O-NO2− labeling experiment, five levels of 18O-NO2− (4 to 539‰) were made by using the O atom exchange between NO2− and H218O. In each treatment, 20 mL of sample was dispensed into 60 -mL serum bottles and sealed with 20-mm butyl stoppers and aluminum crimp seals (Wheaton, United States). The 18O-labeled substrates were injected into the serum bottles. Both the NH4+ and NO2− were set at 20 μmol L−1 in each incubation. For 18O2 labeled incubations, after 18O2 injection, the bottles were shaken at ~120 rpm for 15 min to equilibrate the 18O2 with the dissolved oxygen (DO) in water. The incubation was performed at 30°C in the dark and terminated by adding 1 mL of 10 mol L−1 NaOH. A time-course (0, 6, 12, 24 h) incubation was performed in selected tracer treatments (δ18O-H2O of 129‰; δ18O-O2 of 110‰; δ18O-NO2− of 276‰), and the remaining treatments were terminated at 0 and 24 h; all experiments were performed in triplicates at each time point. An additional set of experiments was performed to examine the rate of abiotic O atom exchange between NO2− and H2O. Briefly, NH4+ and NO2− were added into ~10 mL of fresh medium to a concentration of 20 μmol L−1, and ~0.1 mL of 18O-H2O stock was added to get δ18O-H2O of ~76‰. The incubation was performed at 30°C in the dark and terminated by adding 500 μL of 10 mol L−1 of NaOH at 0, 6, 12, and 24 h with duplicates at each time point.
Sample Analysis.
The samples for NH4+ and NO2− concentration measurement were stored at −20°C until analysis. The concentration of NH4+ and NO2− was measured by colorimetric methods with an AA3 nutrient analyzer or a spectrophotometer. The detection limit for NH4+ and NO2− was 0.5 and 0.03 μmol L−1, and the analytical precision was better than ±3% and ±1%, respectively (37).
The N2O samples were stored at 4°C after incubation. For the preservative test and experiments 3 and 4, before measurements, 1.5 nmol of N2O of known isotope composition (δ15N = −3.2 ± 0.1‰ relative to air N2, δ18O = 36.6 ± 0.1‰ relative to Vienna Standard Mean Ocean Water) was introduced into each serum bottle to provide enough mass for isotopic analysis. For experiments 1, 2, and 5, the samples were measured directly without N2O carrier addition. Concentration and isotopes of N2O were measured using a modified Gas Chromatograph-Isotope Ratio Mass Spectrometer (GC-IRMS) (38). Briefly, two needles were used for He pressurization and N2O purging. For the 20-mL bottles, sample was purged for 6.7 min at a flow rate of 40 mL min−1, and for 60-mL bottles, the purge time was 30 min. The extracted gases were passed through an ethanol trap with dry ice and a chemical trap filled with magnesium perchlorate and Ascarite to remove H2O and CO2. N2O was trapped by liquid nitrogen twice for purification and concentration and then injected into the GC-IRMS with He as carrier gas. N2O mass was determined by ion peak area [m/z of 44, 45, 46] with standard gases of 199.6, 501.0, and 1,000.2 ppmv N2O/He, which were run at ten sample intervals. The precision of this method for N2O mass measurement was estimated to be better than ±3%. δ15N and δ18O were calibrated against two reference tanks (R1: 199.6 ppmv N2O/He, δ15N = −3.2 ± 0.1‰, δ18O = 36.6 ± 0.1‰; R2: 501.0 ppmv N2O/He, δ15N = −1.6 ± 0.1‰, δ18O = 36.6 ± 0.3‰). The precision of δ15N and δ18O measurements with 2 nmol N2O reference gas was better than 0.3‰ and 0.4‰, respectively (n = 20) (30). All the samples were measured within 2 wk after the incubations.
After N2O measurement, the samples were stored at 4°C before further analysis. δ15N and δ18O of NO2− were determined using the bacterial denitrifier method (39, 40) using a Thermo Finnigan Gasbench system with cryogenic extraction and purification system interfaced to a Delta VPLUS isotopic ratio mass spectrometer. Briefly, ~5 to10 nmol of NO2− was quantitatively converted to N2O using the bacterial strain Pseudomonas aureofaciens. The produced N2O was then introduced to the GC-IRMS through an online N2O cryogenic extraction and purification system. δ15N of NO2− values were calibrated against NO3− isotope standards USGS 34, IAEA N3, and USGS 32; δ18O of NO2− values were calibrated against NO3− isotope standards USGS 34, IAEA N3 and USGS 35. The standards were run before, after, and at ten sample intervals. Because of the different branching effect during NO3− and NO2− reduction by P. aureofaciens (i.e., 38‰ vs. 12‰), the δ18O of NO2− was further calibrated by taking account of the branching effect between NO3− and NO2− (26‰) (41). Accuracy (pooled SD) was better than ±0.2‰ for δ15N and ±0.4‰ for δ18O according to analyses of these standards with an injection of a similar amount of NO3−. Quality control was also conducted by analyzing laboratory working reference material (3,000 m deep sea water from the South China Sea).
δ18O of H2O was measured following McIlvin and Casciotti (2006) (42) using the full exchange of O atom between H2O and NO2− under acidic conditions (pH: 6) at room temperature (~25°C) for 2 wk. δ18O of NO2− was measured as described above, and δ18O of H2O was calculated based on the isotope effect of 13‰ between the O atom exchange at room temperature (33).
δ18O of O2 was not measured directly. The δ18O of the 18O2 tracer was calculated from the mixing ratio of air (assuming δ18O air O2 is 24‰) and 18O2 tracer. Briefly, during our incubation, the DO concentration in the medium was near equilibration with air (~244 μmol L−1); thus, a total of ~348 μmol of O2 was present in the bottle (20 mL of medium and 40 mL of air in the headspace). During the 18O-O2 labeling incubation, 0 to 12 μL of 18O2 gas was introduced into the headspace and was then fully equilibrated with the water to attain different enrichments of 18O in the incubation, and the δ18O was then calculated from the 18O/16O after tracer addition.
Calculations.
Rate of labeled N2O production from the 15N-labeled substrate was calculated based on the accumulation of 45N2O (single labeled) and 46N2O (double labeled) during the incubation. Total labeled N2O production rate was defined as the 15N from both 45N2O and 46N2O (Eq. 1). 15NH4+ oxidation rate was calculated from the increase of 15NO2−.
| [1] |
where the total 15N-N2O includes 15N atom from 45N2O (one 15N atom) and 46N2O (two 15N atoms in each molecule).
δ18O of the produced N2O during the incubation was calculated using a two-endmember mixing model (Eq. 2) (43).
| [2] |
where δ18O-N2OP, δ18O-N2Ot24, and δ18O-N2Ot0 denote the δ18O-N2O value of the net produced N2O during the 18O-labeling incubation, δ18O-N2O at the end and beginning of incubation, respectively. Mt24 and Mt0 denote the measured N2O mass at the end and beginning of incubation, respectively.
δ18O of the produced NO2− during the 18O-H2O and 18O-O2 incubations was calculated using Eq. 2 after calibrating the abiotic O atom exchange between NO2− and H2O (9, 33). Briefly, the abiotic O atom exchange rate was derived from the time-course experiment using cell-free medium, which was then used to calibrate the contribution of abiotic O atom exchange during our incubation (Eq. 3). The difference between the measured 18O-NO2− and the predicted 18O-NO2− by abiotic exchange was then used to calculate the δ18O of newly produced NO2− during the incubation. The slopes of the newly produced δ18O-NO2− and δ18O-N2O against δ18O of different substrates (H2O, O2, NO2−) were identified as the fraction contribution of O atom from various substrates to the NO2− and N2O (9, 33).
| [3] |
where δ18O-NO2−abio, δ18O-NO2−t0, and δ18O-NO2−eq are δ18O value of NO2− at the end, beginning, and the equilibrated NO2− with H2O due to abiotic O exchange. t is the incubation length in hours and k is the rate constant.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We gratefully acknowledge advice, scientific discussions, and comments on the manuscript from Michael L. Bender. We appreciate Xiangpeng Li, Long Q. Ngo, Li Liu, Junyi Ni, and Tingyuan Liu’s assistance during cell culture and harvest, Lili Han for the nutrient measurements, and Sergey Oleynik for maintaining the mass spectrometers at Princeton. This work was funded by the Simons Foundation through award No. 675459 to B.B.W. and was supported by National Natural Science Foundation of China through grants 42125603, 92058204, and 41890802 and by the start-up funding of the University of Oklahoma to W.Q.
Author contributions
X.S.W., W.Q., and B.B.W. designed research; X.S.W., L.H., H.-X.S., H.S., and S.T. performed research; S.-J.K., and Y.Z. contributed new reagents/analytic tools; X.S.W., L.H., W.Q., and B.B.W. analyzed data; and X.S.W. and B.B.W. wrote the paper with input from all authors.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Xianhui S. Wan, Email: xianhuiw@princeton.edu.
Bess B. Ward, Email: bbw@princeton.edu.
Data, Materials, and Software Availability
All data needed to evaluate the conclusions in the paper are deposited in Zenodo database that can be accessed through (https://doi.org/10.5281/zenodo.7378577) (44).
Supporting Information
References
- 1.Santoro A. E., Richter R. A., Dupont C. L., Planktonic marine Archaea. Ann. Rev. Mar. Sci. 11, 131–158 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Martens-Habbena W., Berube P. M., Urakawa H., de la Torre J. R., Stahl D. A., Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461, 976–979 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Buitenhuis E. T., Suntharalingam P., Le Quéré C., Constraints on global oceanic emissions of N2O from observations and models. Biogeosciences 15, 2161–2175 (2018). [Google Scholar]
- 4.Freing A., Wallace D. W. R., H. W. Bange, Global oceanic production of nitrous oxide. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 1245–1255 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji Q., Buitenhuis E., Suntharalingam P., Sarmiento J. L., Ward B. B., Global nitrous oxide production determined by oxygen sensitivity of nitrification and denitrification. Global Biogeochem. Cy. 32, 1790–1802 (2018). [Google Scholar]
- 6.Walker C. B., et al. , Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc. Natl. Acad. Sci. U.S.A. 107, 8818–8823 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hink L., et al. , Kinetics of NH3-oxidation, NO-turnover, N2O-production and electron flow during oxygen depletion in model bacterial and archaeal ammonia oxidisers. Environ. Microbiol. 12, 4882–4896 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Qin W., et al. , Influence of oxygen availability on the activities of ammonia-oxidizing archaea. Environ. Microbiol. Rep. 9, 250–256 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Santoro A. E., Buchwald C., McIlvin M. R., Casciotti K. L., Isotopic signature of N2O produced by marine ammonia-oxidizing archaea. Science 333, 1282–1285 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Prosser J. I., Hink L., Gubry-Rangin C., Nicol G. W., Nitrous oxide production by ammonia oxidizers: Physiological diversity, niche differentiation and potential mitigation strategies. Glob. Chang. Biol. 26, 103–118 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Stein L. Y., Insights into the physiology of ammonia-oxidizing microorganisms. Curr. Opin. Chem. Biol. 49, 9–15 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Stein L. Y., et al. , Comment on“A critical review on nitrous oxide production by ammonia-oxidizing Archaea” by Lan Wu, Xueming Chen, Wei Wei, Yiwen Liu, Dongbo Wang, and Bing-Jie Ni. Environ. Sci. Technol. 55, 797–798 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Kozlowski J. A., Stieglmeier M., Schleper C., Klotz M. G., Stein L. Y., Pathways and key intermediates required for obligate aerobic ammonia-dependent chemolithotrophy in bacteria and Thaumarchaeota. ISME J. 10, 1836–1845 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martens-Habbena W., et al. , The production of nitric oxide by marine ammonia-oxidizing archaea and inhibition of archaeal ammonia oxidation by a nitric oxide scavenger. Environ. Microbiol. 17, 2261–2274 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Vajrala N., et al. , Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine archaea. Proc. Natl. Acad. Sci. U.S.A. 110, 1006–1011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung M. Y., et al. , Indications for enzymatic denitrification to N2O at low pH in an ammonia-oxidizing archaeon. ISME J. 13, 2633–2638 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraft B., et al. , Oxygen and nitrogen production by an ammonia-oxidizing archaeon. Science 375, 97–100 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Stieglmeier M., et al. , Aerobic nitrous oxide production through N-nitrosating hybrid formation in ammonia-oxidizing archaea. ISME J. 8, 1135–1146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kool D. M., Müller C., Wrage N., Oenema O., Van Groenigen J. W., Oxygen exchange between nitrogen oxides and H2O can occur during nitrifier pathways. Soil Biol. Biochem. 8, 1632–1641 (2009). [Google Scholar]
- 20.Wrage N., van Groenigen J. W., Oenema O., Baggs E. M., A novel dual-isotope labeling method for distinguishing between soil sources of N2O. Rapid Commun. Mass Spectrom. 19, 3298–3306 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Liu S., et al. , Abiotic conversion of extracellular NH2OH contributes to N2O emission during ammonia oxidation. Environ. Sci. Technol. 51, 13122–13132 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Gruber N., “The marine nitrogen cycle: Overview and challenges” in Nitrogen in the Marine Environment, D. G. Capone, D. A. Bronk, M. R. Mulholland, E. J. Carpenter, Eds. (Elsevier, ed. 2, 2008), pp. 1–50. [Google Scholar]
- 23.Hernández-León S., Fraga C., Ikeda T., A global estimation of mesozooplankton ammonium excretion in the open ocean. J. Plankton Res. 30, 577–585 (2008). [Google Scholar]
- 24.Breider F., et al. , Response of N2O production rate to ocean acidification in the western North Pacific. Nat. Clim. Chang. 12, 954–958 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charpentier J., Farias L., Yoshida N., Boontanon N., Raimbault P., Nitrous oxide distribution and its origin in the Central and Eastern South Pacific Subtropical Gyre. Biogeosciences 4, 729–741 (2007). [Google Scholar]
- 26.Popp B. N., et al. , Nitrogen and oxygen isotopomeric constraints on the origins and sea-to-air flux of N2O in the pligotrophic Subtropical North Pacific Gyre. Global Biogeochem. Cy. 16, 1064 (2002). [Google Scholar]
- 27.Zhang G. L., et al. , Distribution of concentration and stable isotopic composition of N2O in the shelf and slope of the Northern South China Sea: Implications for production and emission. J. Geophys. Res. Oceans 124, 6218–6234 (2019). [Google Scholar]
- 28.Frey C., et al. , Regulation of nitrous oxide production in low-oxygen waters off the coast of Peru. Biogeosciences 17, 2263–2287 (2020). [Google Scholar]
- 29.Ji Q., Ward B. B., Nitrous oxide production in surface waters of the mid-latitude North Atlantic Ocean. J. Geophys. Res. Oceans 122, 2612–2621 (2017). [Google Scholar]
- 30.Wan X. S., et al. , Epipelagic nitrous oxide production offsets carbon sequestration by the biological pump. Nat. Geosci. 16, 29–36 (2023). [Google Scholar]
- 31.Frey C., et al. , Kinetics of nitrous oxide production from ammonia oxidation in the Eastern Tropical North Pacific. Limnol. Oceanogr. 68, 424–438 (2022), 10.1002/lno.12283. [DOI] [Google Scholar]
- 32.Korth F., Kock A., Arevalo-Martinez D. L., Bange H. W., Hydroxylamine as a potential indicator of nitrification in the open ocean. Geophys. Res. Lett. 46, 2158–2166 (2019). [Google Scholar]
- 33.Buchwald C., Casciotti K. L., Isotopic ratios of nitrite as tracers of the sources and age of oceanic nitrite. Nat. Geosci. 6, 308–313 (2013). [Google Scholar]
- 34.Casciotti K. L., McIlvin M., Buchwald C., Oxygen isotopic exchange and fractionation during bacterial ammonia oxidation. Limnol. Oceanogr. 55, 753–762 (2010). [Google Scholar]
- 35.Qin W., et al. , Marine ammonia-oxidizing archaeal isolates display obligate mixotrophy and wide ecotypic variation. Proc. Natl. Acad. Sci. U.S.A. 111, 12504–12509 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amin S. A., et al. , Copper requirements of the ammonia-oxidizing archaeon Nitrosopumilus maritimus SCM1 and implications for nitrification in the marine environment. Limnol. Oceanogr. 58, 2037–2045 (2013). [Google Scholar]
- 37.Han A., et al. , Nutrient dynamics and biological consumption in a large continental shelf system under the influence of both a river plume and coastal upwelling. Limnol. Oceanogr. 57, 486–502 (2012). [Google Scholar]
- 38.McIlvin M. R., Casciotti K. L., Fully automated system for stable isotopic analyses of dissolved nitrous oxide at natural abundance levels. Limnol. Oceanogr.-Meth. 8, 54–66 (2010). [Google Scholar]
- 39.Casciotti K. L., Sigman D. M., Hastings M. G., Böhlke J. K., Hilkert A., Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal. Chem. 74, 4905–4912 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Weigand M. A., Foriel J., Barnett B., Oleynik S., Sigman D. M., Updates to instrumentation and protocols for isotopic analysis of nitrate by the denitrifier method. Rapid Commun. Mass Spectrom. 30, 1365–1383 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Casciotti K. L., Böhlke J. K., McIlvin M. R., Mroczkowski S. J., Hannon J. E., Oxygen isotopes in nitrite: Analysis, calibration, and equilibration. Anal. Chem. 79, 2427–2436 (2007). [DOI] [PubMed] [Google Scholar]
- 42.McIlvin M. R., Casciotti K. L., Method for the Analysis of δ18O in Water. Anal. Chem. 78, 2377–2381 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Fry B., Steady state models of stable isotopic distributions. Isotopes. Environ. Health. Stud. 39, 219–232 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Wan X. S., et al. , Dataset of SCM1 N2O production. Zenodo (2022). 10.5281/zenodo.7378577. (Deposited 29 November 2022). [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All data needed to evaluate the conclusions in the paper are deposited in Zenodo database that can be accessed through (https://doi.org/10.5281/zenodo.7378577) (44).