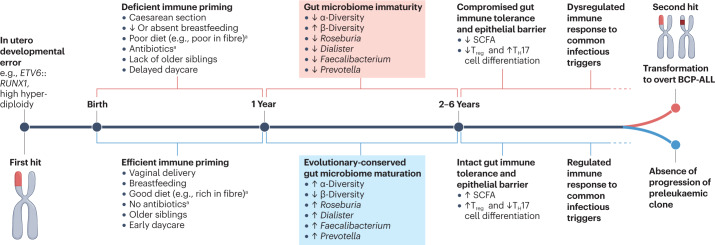
Fig. 4. The two-hit model of childhood B cell precursor-acute lymphoblastic leukaemia.
Proposed role of the gut microbiome in the two-hit model of childhood B cell precursor-acute lymphoblastic leukaemia (BCP-ALL) based on the results of currently available preliminary studies analysed in the present work. Chromosomal first hits are necessary events for the development of BCP-ALL, but are not sufficient to drive leukaemogenesis. The synergistic effect of adverse early-life exposures, such as caesarean section, reduced or absent breastfeeding, reduced dietary fibrea, antibioticsa, lack of older siblings and delayed entry into daycare, may lead to gut microbiome immaturity during a critical time in the development of the immune system. A deficiency in short-chain fatty acid (SCFA)-producing bacterial taxa can compromise gut microbiome-mediated immune priming leading to suppression of regulatory T (Treg) cells and promotion of T helper 17 (TH17)-dominated immune responses. SCFA deficiency can also compromise the integrity of the gut epithelial barrier and facilitate the systemic translocation of opportunistic pathogens, as well as increase susceptibility to viral infections. The resulting dysregulated pro-inflammatory immune responses against common infectious triggers can ultimately lead to an increased risk of leukaemic transformation in a small proportion (about 1%) of children with in utero-acquired preleukaemic clones. ETV6::RUNX1, ETS translocation variant 6::runt-related transcription factor 1. aThese variables have not been systematically evaluated as risk factors for ALL.