Fig. 1.
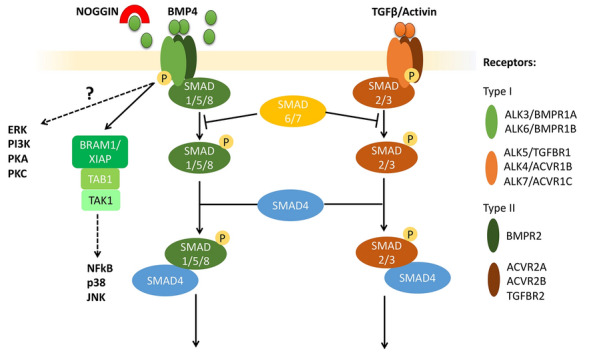
BMP and TGFB/INHBA (Activin) branches of the TGFB signaling pathway. Ligands of the TGFB super-family activate Type I and Type II receptors, transmembrane serine/threonine kinases, classified into five main type I (ALK3/BMPR1A, ALK4/ACVR1B, ALK5/TGFBR1, ALK6/BMPR1B, and ALK7/ACVR1C), and three type II (BMPR2, ACVR2A, and ACVR2B) receptors. BMP4 specifically binds homodimers of ALK3/BMPR1A or ALK6/BMPR1B, which subsequently recruit type II homodimers forming a tetramer complex. This interaction leads to the activation of both the “canonical”, or SMAD-dependent, and the “non-canonical”, or SMAD-independent, signaling pathways. In the canonical pathway, recruitment of type II receptors leads to the phosphorylation of Type I receptors, which then phosphorylate receptor-regulated SMAD (R-SMAD) 1/5/8 proteins. Activated SMAD-1/5/8 binds to SMAD4 and translocates into the nucleus, where it mediates the transcription of BMP-specific target genes. The “non-canonical” pathway activates various signaling cascades in a SMAD-independent manner, including those involving ERK, TAK1-p38, PI3K/AKT, and PKC. The SMAD-independent activation of TAK1, upstream of p38, JNK, and NFkB, is known to be mediated by TAB1 (TAK-binding protein 1) via the complex with BRAM1 (BMP Receptor Associated Molecule 1) or XIAP (X-linked inhibitor-of-apoptosis protein). NOGGIN is a well-known inhibitor of the BMP signaling pathway, acting by sequestering ligands and preventing their interaction with receptors. TGFB and Activin bind to specific Type I and II receptors, which phosphorylate R-SMAD 2/3. Inhibitory SMAD 6/7 interacts with both branches by preventing phosphorylation of R-SMADs
