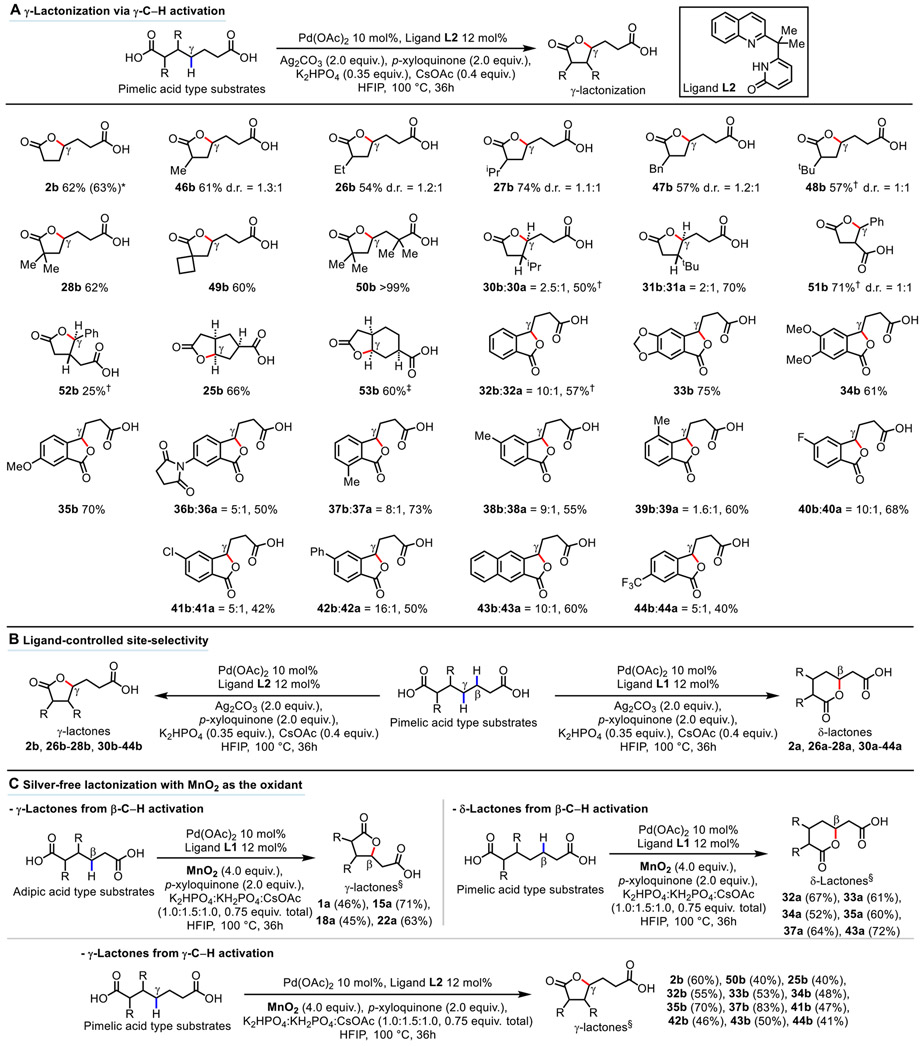
Fig. 3. Substrate scope of γ-lactones from γ-C─H activation, demonstration of switchable site-selectivity, and examples of silver-free lactonization reactions with MnO2 as the oxidant.
(A) γ-Lactonization via γ-C─H activation. Reaction conditions: Substrate (0.1 mmol), Pd(OAc)2 10 mol%, Ligand L2 12 mol%, Ag2CO3 (2.0 equiv.), p-xyloquinone (2.0 equiv.), K2HPO4 (0.35 equiv.), CsOAc (0.4 equiv.), HFIP (1.0 mL), 100 °C, 36h. Isolated yields are reported. *Reaction conducted with 1.0 g of pimelic acid 2. †Isolated as the corresponding benzyl ester. ‡Based on reactive diastereomer (see Supplementary Material p. S92-S93 for explanation). (B) Demonstration of switchable site-selectivity with 19 examples. (C) Examples of silver-free lactonization with MnO2 as the oxidant (22 examples). Reaction conditions: Substrate (0.1 mmol), Pd(OAc)2 10 mol%, Ligand L1 or L2 12 mol%, MnO2 (4.0 equiv.), p-xyloquinone (2.0 equiv.), K2HPO4:KH2PO4:CsOAc (1.0:1.5:1.0, 0.75 equiv. in total), HFIP (1.0 mL), 100 °C, 36h. §NMR yields.