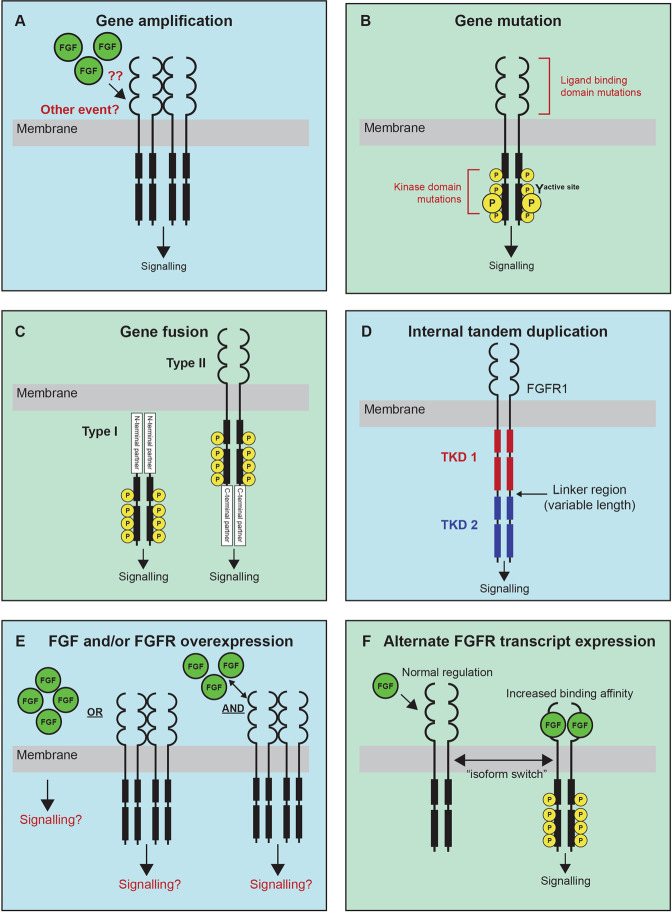
Fig. 3. Mechanisms of FGFR kinase activation.
A Gene amplification is predicted to result in an increase in FGFR protein expression and increased FGFR signalling. It is likely other events, including an increase in FGF ligand expression, cooperate with FGFR amplification to drive receptor activation. B Mutations in FGFRs most commonly occur in the ligand binding or kinase domain which result in increased ligand-binding affinity or ligand-independent receptor activation through a disruption of normal kinase autophosphorylation, respectively, leading to FGFR activation. C There are two classes of fusion genes which involve FGFRs, Type I –fusion of an N-terminal partner, or Type II—fusion of a C-terminal partner. Both N and C-terminal partners commonly contribute dimerisation domains that result in ligand-independent dimerisation and phosphorylation and activation of the FGFR kinase. D FGFR1 internal tandem duplication (ITD) results in a receptor with containing two tyrosine kinase domains (TKD1 and TKD2) separated by a linker region of variable length, which has been shown to promote kinase activation. E The functional result of increased FGF ligand or FGFR RNA or protein overexpression is currently unclear. It is possible that it is the combination of both ligand and receptor overexpression that will promote FGFR activation. F FGFR ligand binding domains are subject to splicing under normal conditions, but alternate isoforms lacking regions of the ligand binding domains have been shown to be upregulated in specific cancers. The common functional result of FGFRs that lack these domains is an increase in either ligand or heparin binding affinity which results in increased FGFR activation.