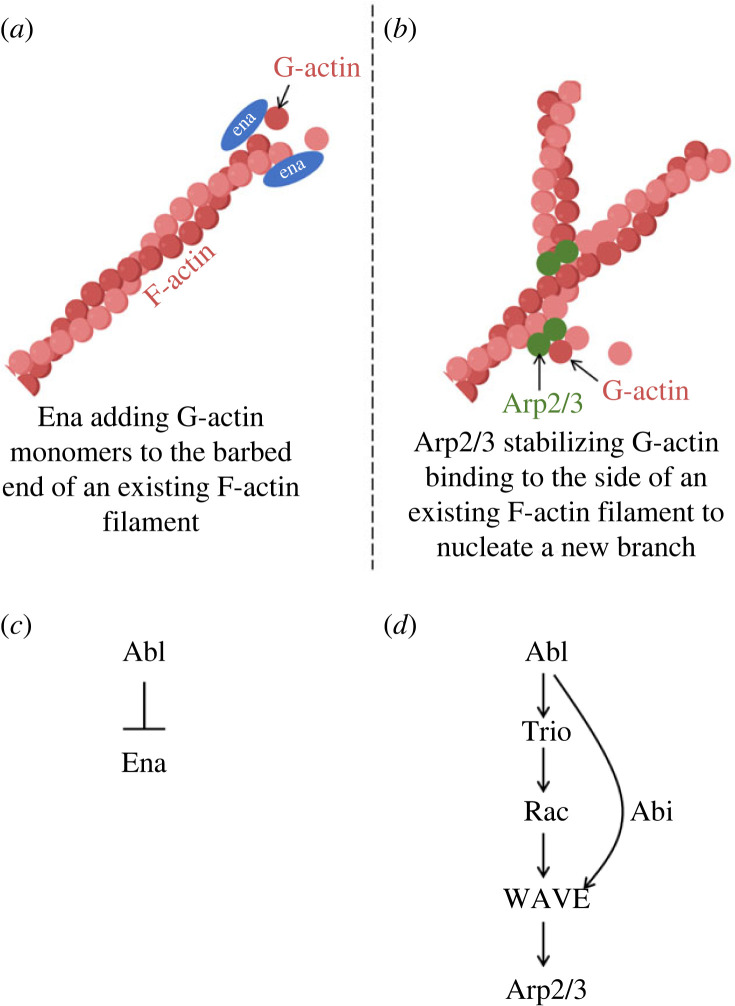
Figure 4.
Schematic depiction of actin polymerization and nucleation by Ena and Arp2/3, respectively, and the relationship of Abl to the activity of each. (a) Ena (blue oval) has both an F-actin and a G-actin binding domain and stimulates actin polymerization in part by transferring a G-actin monomer (red circle) to the barbed end of an existing F-actin filament (depicted as a helix of G-actin monomers). Ena is present in vivo as a tetramer, but for clarity only two monomers of Ena are shown here. (b) Arp2/3 (green circles) nucleates initiation of a new actin filament by binding to the side of an existing filament and providing a site for stable binding of G-actin monomers from solution. Additional monomers can then add to the end of the newly formed filament. (c) Abl negatively regulates Ena activity. This occurs by at least two mechanisms, direct phosphorylation of tyrosine residues on Ena, which is apparently inhibitory for Ena function, and also regulation of Ena localization that excludes Ena from particular regions of the cell. The mechanism by which Abl controls Ena localization is not known. (d) Abl positively regulates Arp2/3 activity. Abl activates the Rac-specific GEF Trio and Rac in turn activates WAVE complex. WAVE then recruits Arp2/3 and stimulates its actin nucleation activity. In parallel, Abl also associates physically with WAVE complex components, notably Abi (depicted as an additional arrow in the diagram).