Figure 1. Participant Flow in a Study of Letermovir vs Valganciclovir for Cytomegalovirus (CMV) Prophylaxis in Kidney Transplant Recipients.
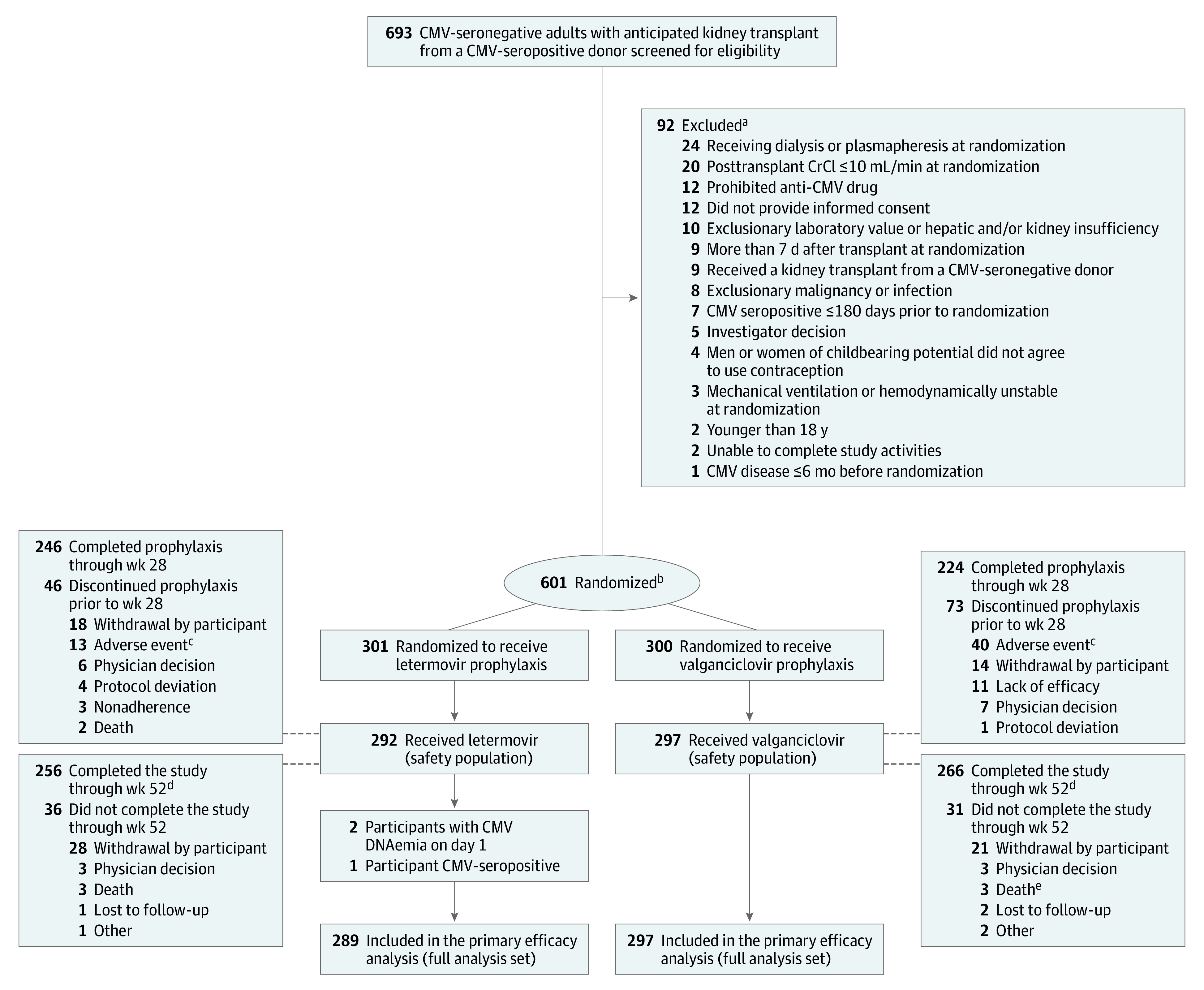
CrCI indicates creatinine clearance.
aParticipants could have ≥1 reason for exclusion. The inclusion and exclusion criteria are listed in Supplement 1.
bStratified by receipt of lymphocyte-depleting induction immunosuppression.
cOne participant developed glomerulonephritis (reported as an adverse event) that began the day after transplant and prior to initiating letermovir prophylaxis. One participant discontinued valganciclovir prophylaxis due to an adverse event (hallucinations) and died prior to week 28.
dParticipants were encouraged to complete all remaining scheduled visits through week 52 after confirmation of CMV disease and/or early discontinuation of prophylaxis.
eOne death due to COVID-19 was not reported as an adverse event.
