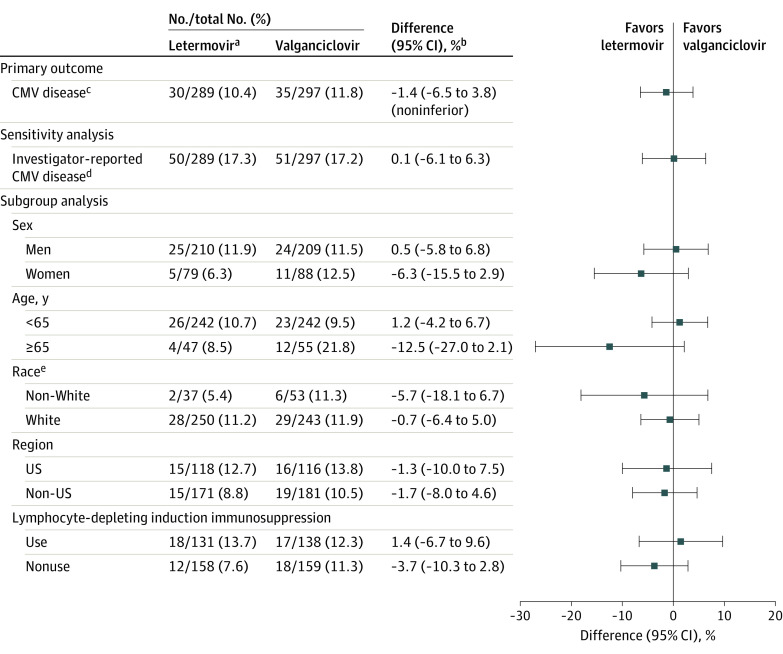
Figure 2. Primary Outcome of Cytomegalovirus (CMV) Disease With Letermovir vs Valganciclovir Prophylaxis Through Week 52 in the Full Analysis Set.
aAll participants in the letermovir group received acyclovir for prophylaxis of herpes simplex and varicella-zoster virus.
bThe 95% CIs for the differences in proportions of participants were calculated using stratum-adjusted Mantel-Haenszel method with the difference weighted by the harmonic mean of sample size per group for each stratum (use/nonuse of lymphocyte-depleting induction immunosuppression). The upper bound of the 2-sided 95% CI for the primary outcome had to be no higher than 10% to conclude noninferiority. Participants who did not complete the study through week 52 or a had missing result for CMV DNAemia in the week-52 visit window were not considered failures (observed failure approach).
cCMV disease confirmed by the independent masked adjudication committee (CMV end-organ disease or CMV syndrome).
dPrespecified sensitivity analysis for investigator-reported CMV disease (included CMV syndrome and/or CMV end-organ disease).
eData were not available for 2 participants in the letermovir group and 1 participant in the valganciclovir group.