Figure 4. Cloning and characterization of ABbA, the broadly blocking human mAb.
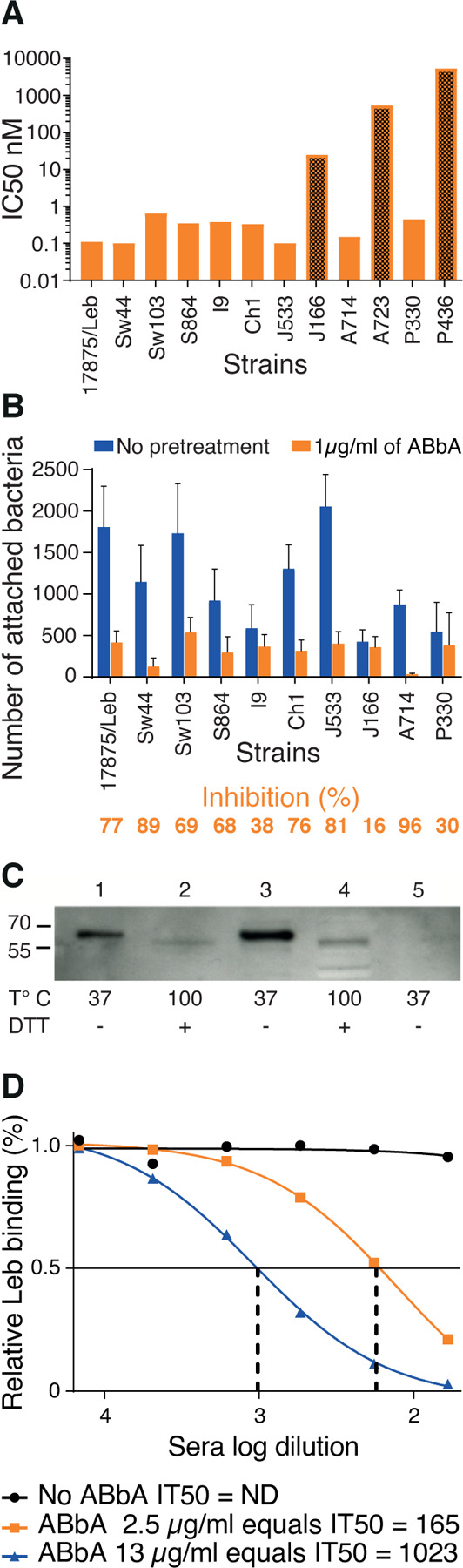
(A) Tests of the ABbA IC50 for the 12 world-wide H. pylori strains from Figure 2A. ABbA blocked Leb binding of 9 strains but was less efficient with J166 (which has low Leb-binding affinity) (Figure S1D) (darker bar 1) and did not block binding of the two Indigenous blood group O-binding American Specialist strains A723 and P436 (darker bars 2 and 3) that both exhibit adaptive substitutions in the CBD that is critical for binding to ABO/Leb (Figure S2C) 13,14,25.
(B) Test of the blocking of H. pylori attachment by ABbA to human gastric mucosa in vitro by the series of H. pylori strains from (A), except for A723 and P436. The reduction in attachment by ABbA inhibition (in orange) and Inhibition (%) closely reflected the ABbA IC50 for the corresponding strains, where the J166 strain with the higher IC50 was similarly modestly blocked by ABbA in terms of attachment (16%).
(C) ABbA-scFv recognized both purified BabA and BabA in size-separated bacterial whole-cell protein extracts (WCPE) under semi-native conditions (Lanes 1 and 3, respectively) but not under denaturing conditions (Lanes 2 and 4, respectively). As a negative control, WCPE of the 17875babA1A2-mutant (with no BabA) was applied under semi-native conditions (Lane 5).
(D) Tests of ABbA concentration in terms of inhibition activity as defined by IT50 were performed by two dilution series, which showed that 1 µg/mL of ABbA equals IT50 ~70.
