FIGURE 1.
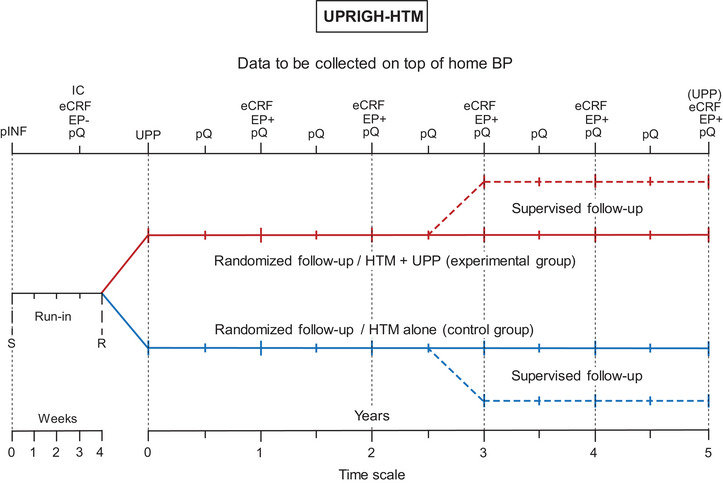
UPRIGHT‐HTM design. eCRF, electronic report forms completed by investigators; EP, absence (−) / incidence (+) of the components of the primary endpoint during the run‐in/follow‐up periods, respectively; IC, written informed consent; pINF, patients received the UPRIGHT‐HTM information sheet and were familiarized with operating the blood pressure monitoring devices; pQ, patient‐administered questionnaires, R, randomization after stratification for center and sex; S, initial screening; UPP, urinary proteomic profiling (mandatory prior to randomization—optional at the end of follow‐up). Reproduced from open‐access reference 5.
