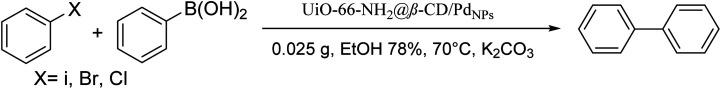
Preparation of various organic compounds by Suzuki coupling reaction under optimum conditionsa.
| |||||
|---|---|---|---|---|---|
| Entry | Aryl boronic acid | Aryl halide | Product | Time (min) | Yield (%) |
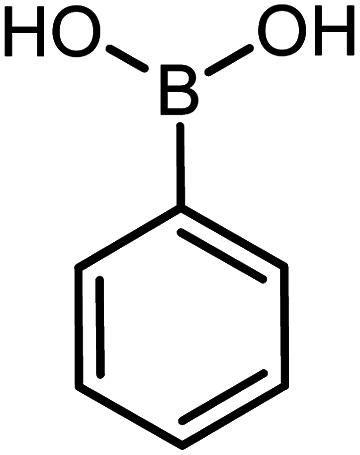
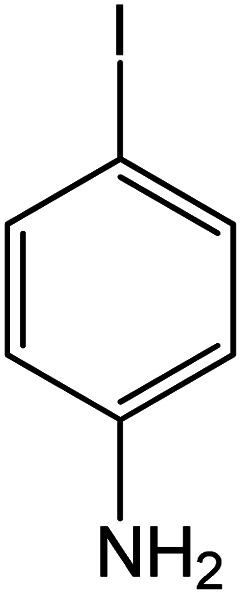
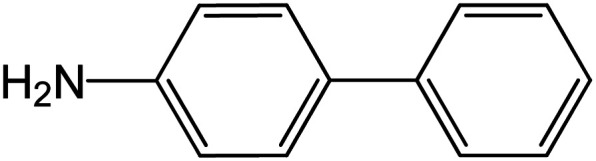
| 1 |
|
|
|
60 | 92 |
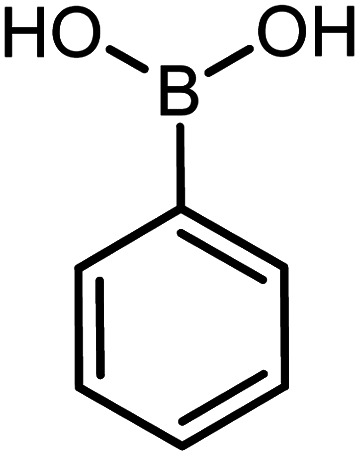
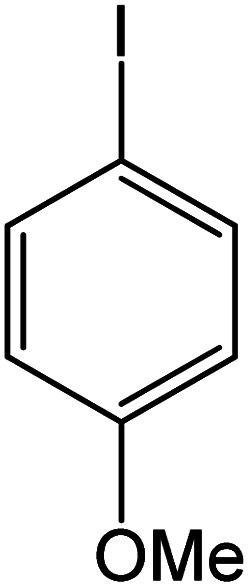
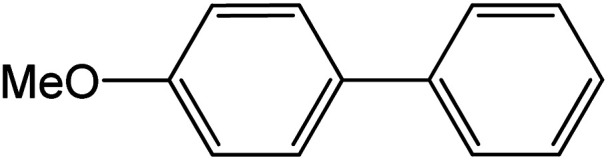
| 2 |
|
|
|
60 | 95 |
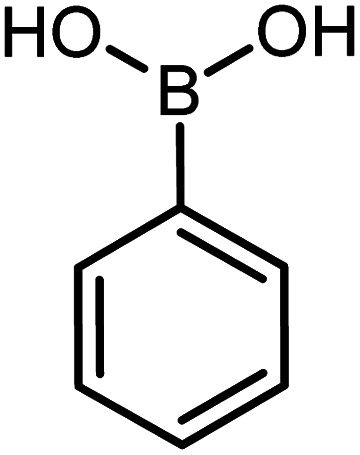
| 3 |
|
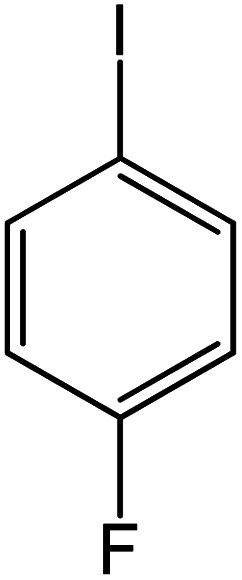
|
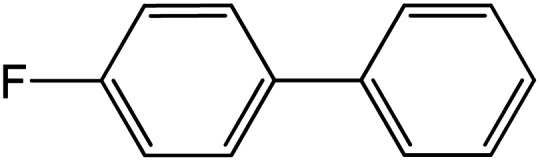
|
60 | 97 |
| 4 |
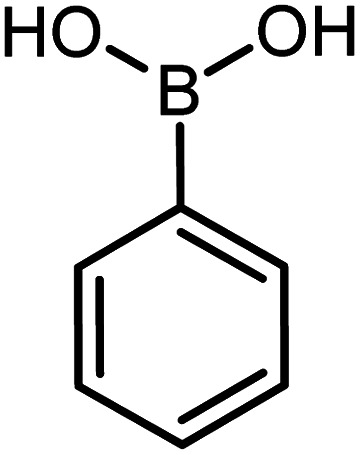
|
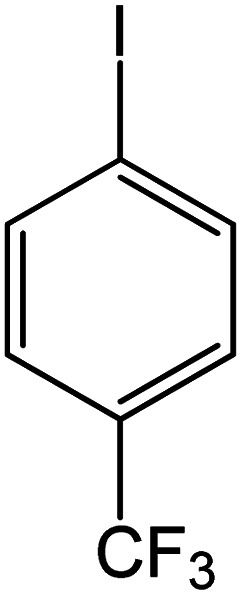
|
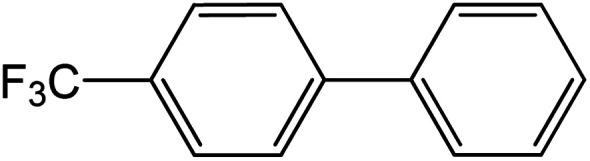
|
60 | 99 |
| 5 |
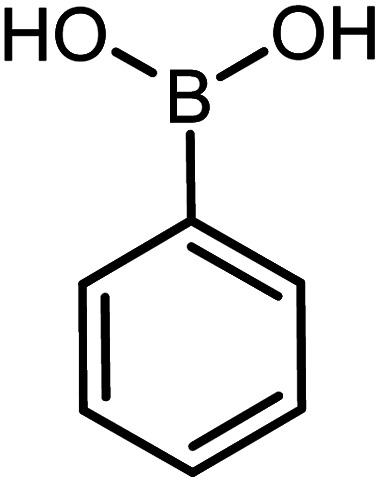
|
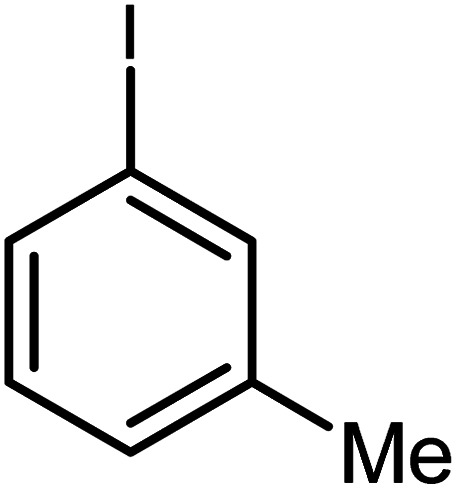
|
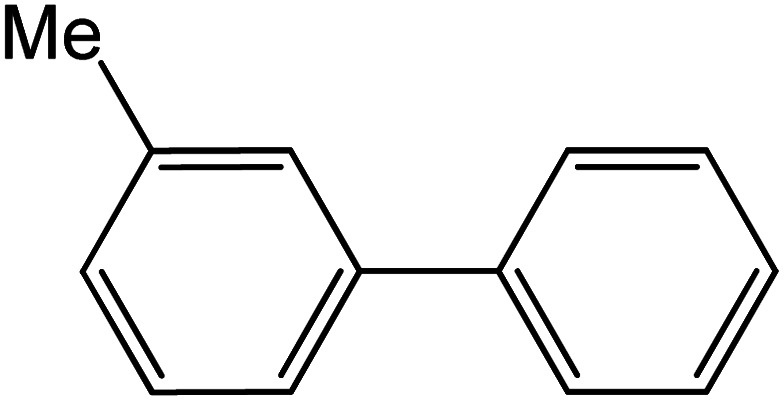
|
60 | 77 |
| 6 |
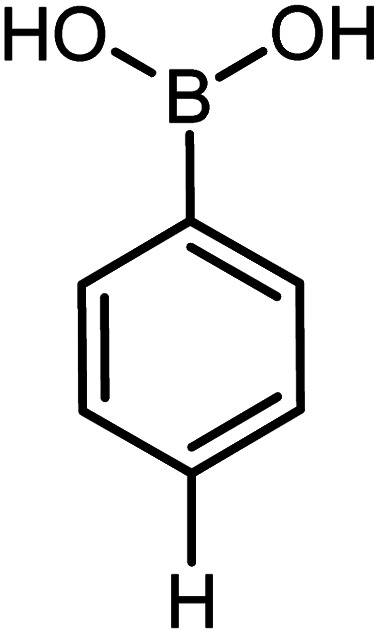
|
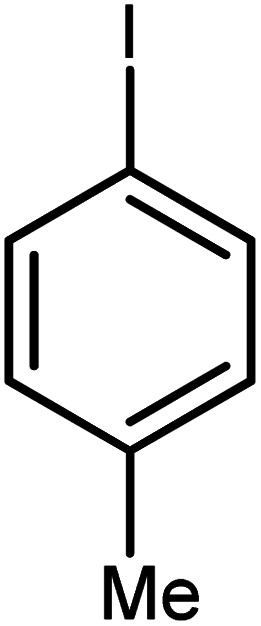
|
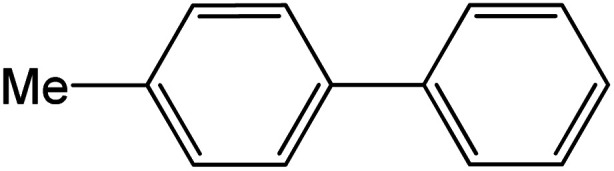
|
92 | 92 |
| 7 |
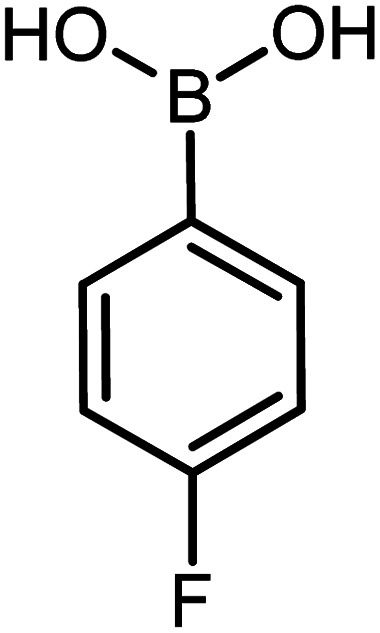
|
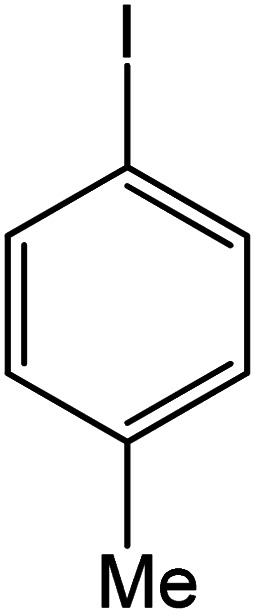
|
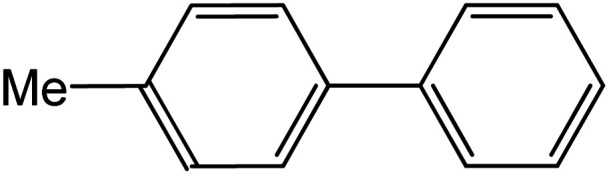
|
60 | 95 |
| 8 |
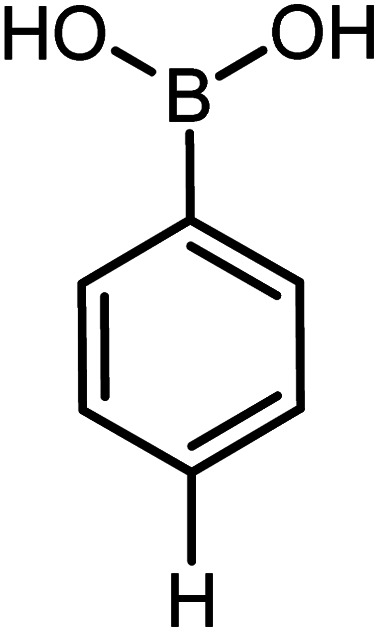
|
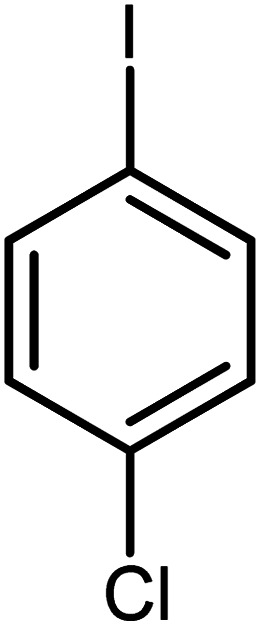
|
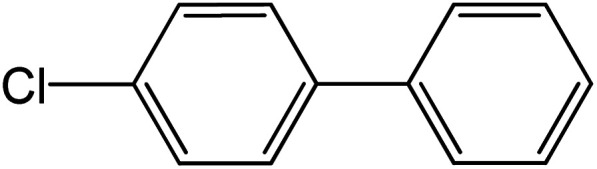
|
60 | 99 |
| 9 |
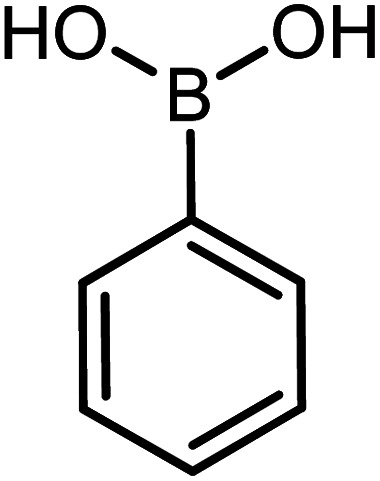
|
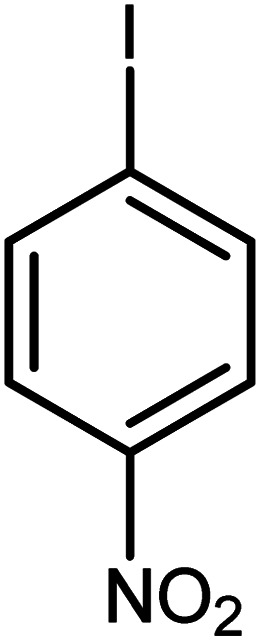
|
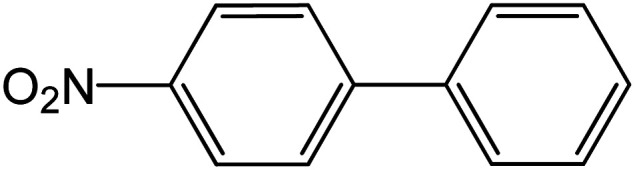
|
60 | 98 |
| 10 |
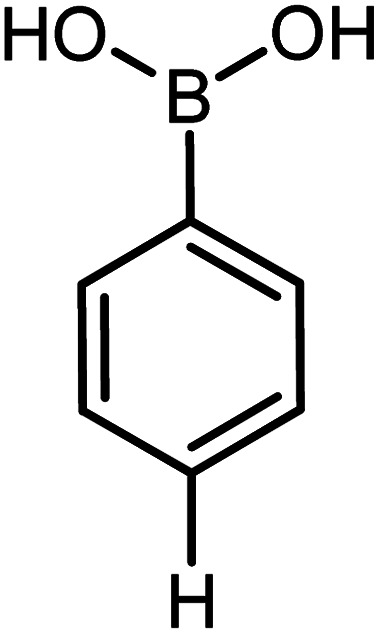
|
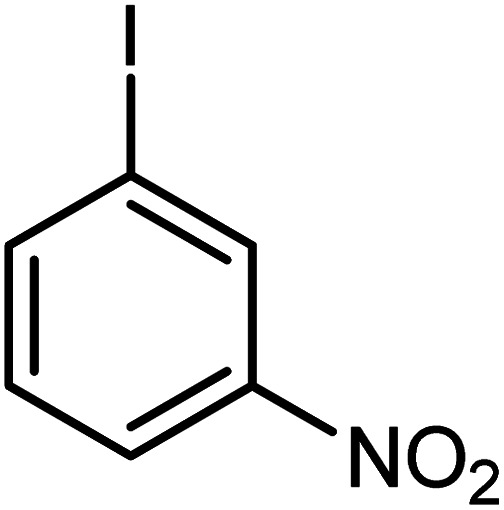
|
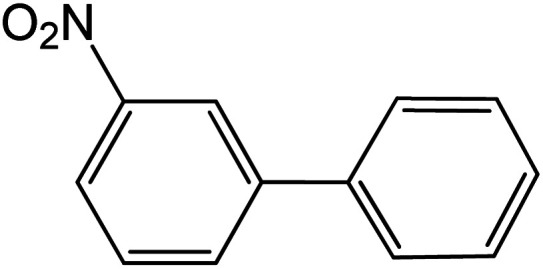
|
60 | 99 |
| 11 |
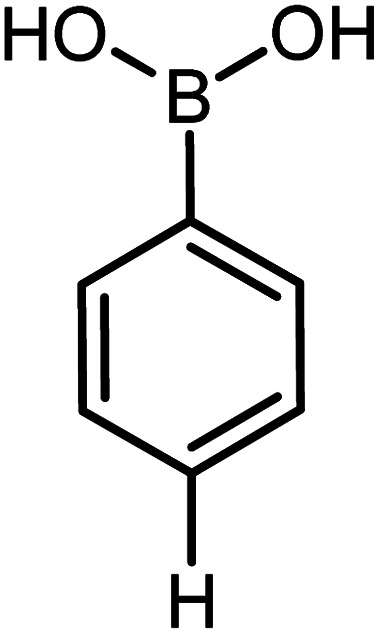
|
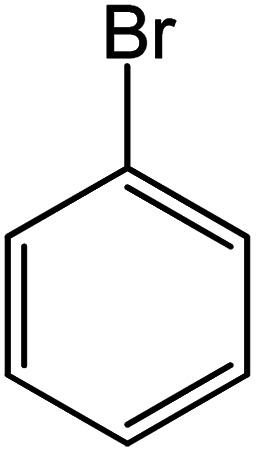
|
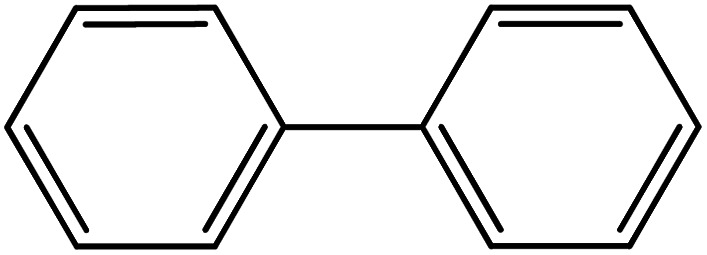
|
60 | 98 |
| 12 |
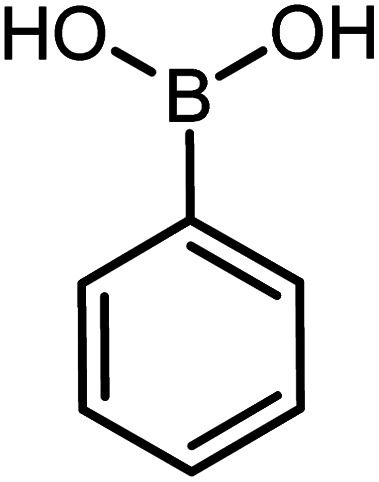
|
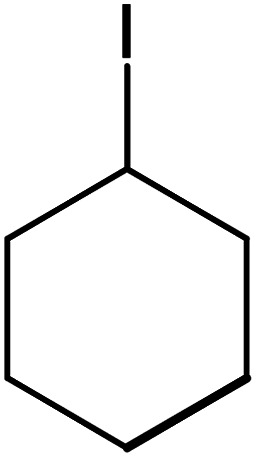
|
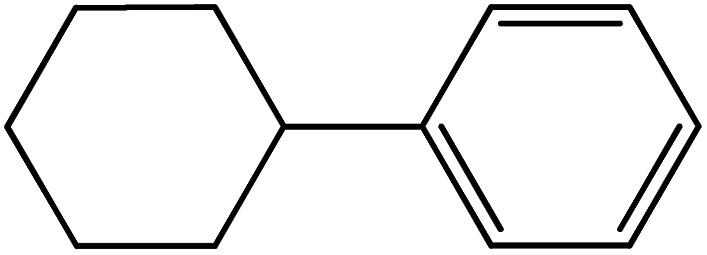
|
73 | 99 |
| 13 |
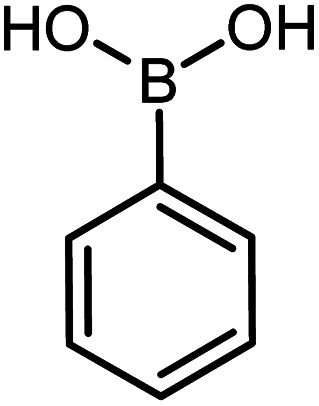
|
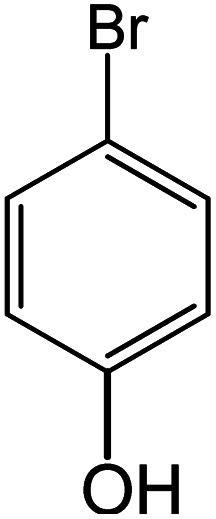
|
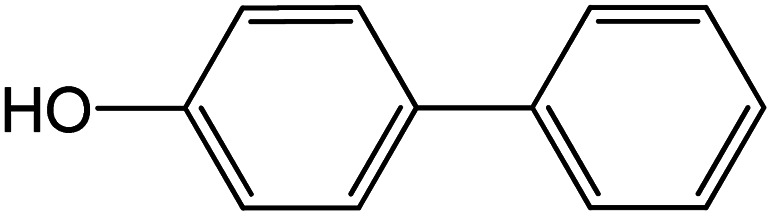
|
60 | 76 |
| 14 |
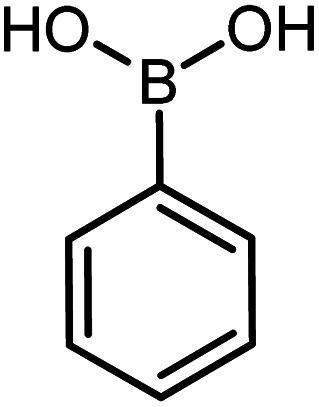
|
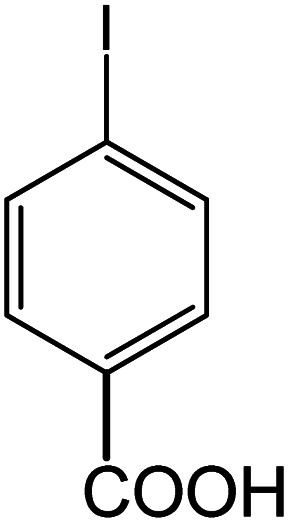
|
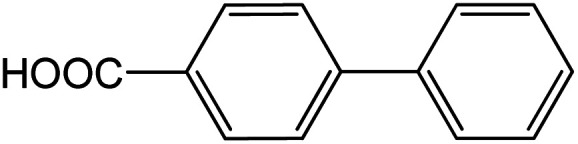
|
60 | 95 |
| 15 |
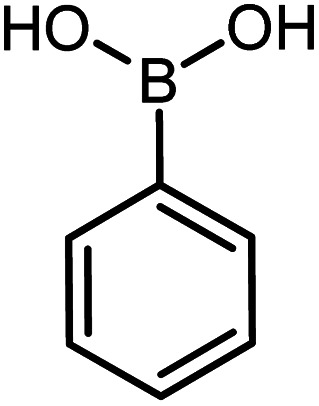
|
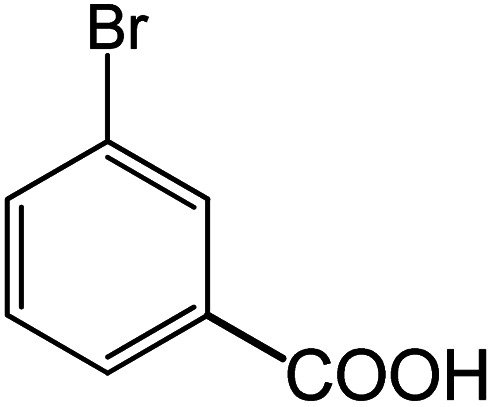
|
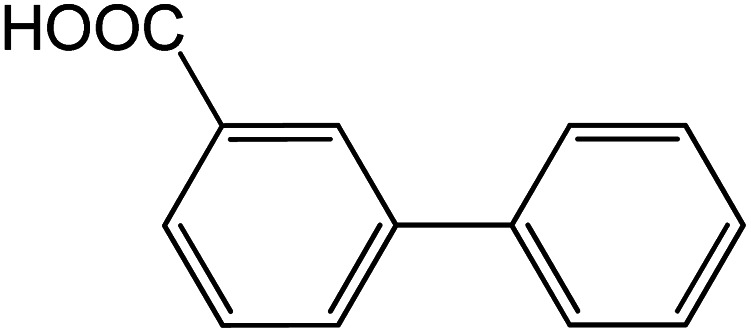
|
60 | 90 |
| 16 |
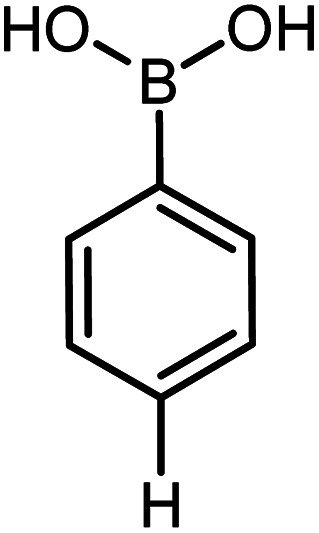
|
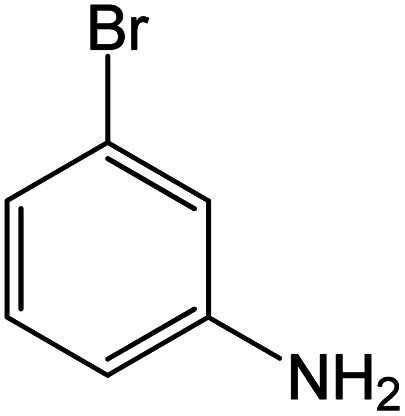
|
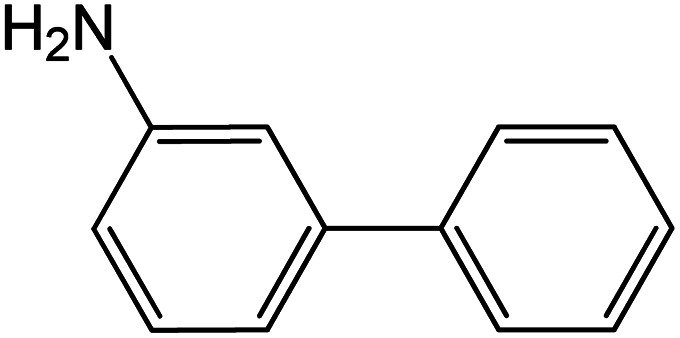
|
60 | 98 |
| 17 |
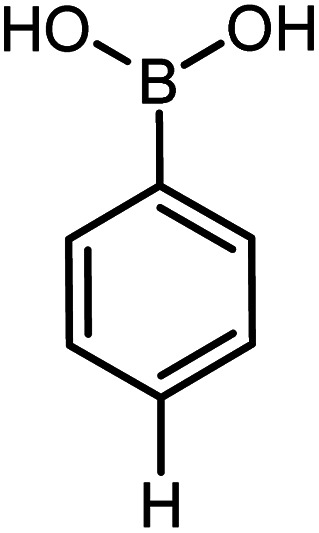
|
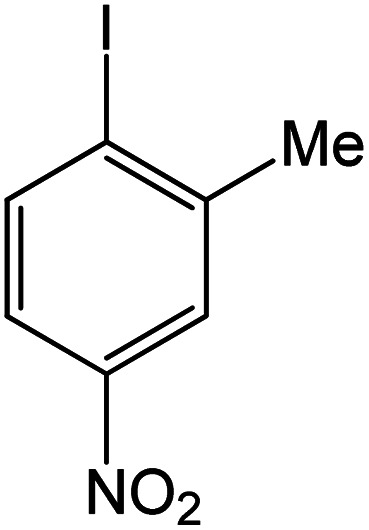
|
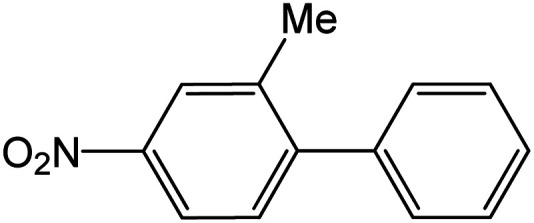
|
60 | 96 |
| 18 |
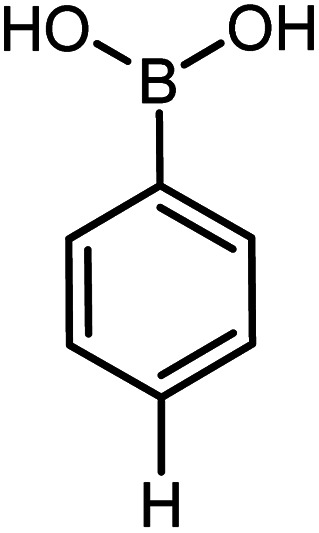
|
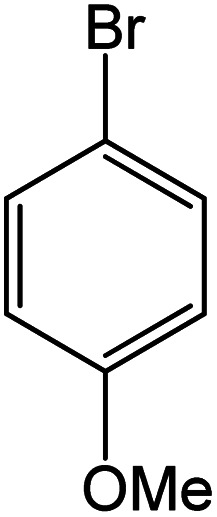
|
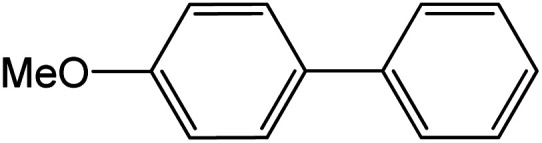
|
90 | 99 |
| 19 |
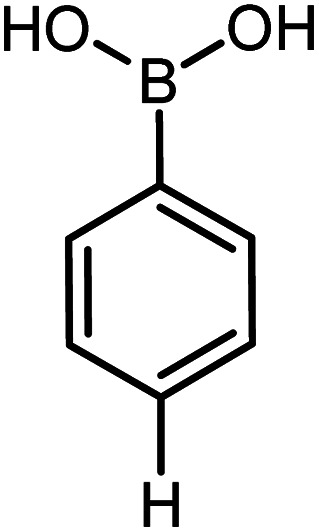
|
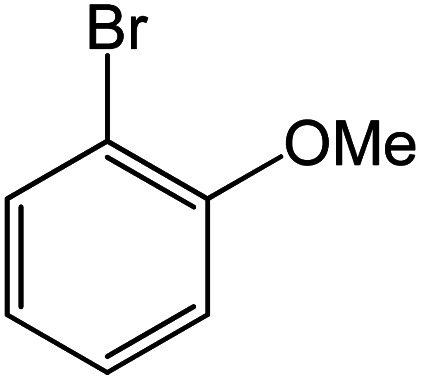
|
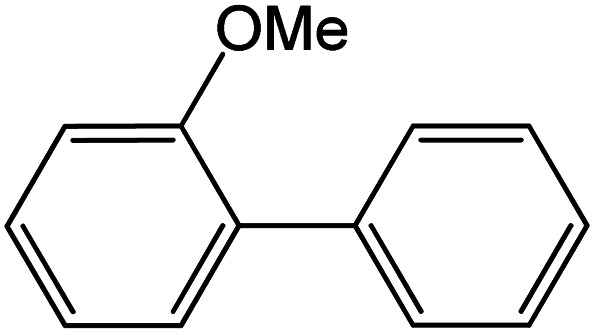
|
60 | 97 |
| 20 |
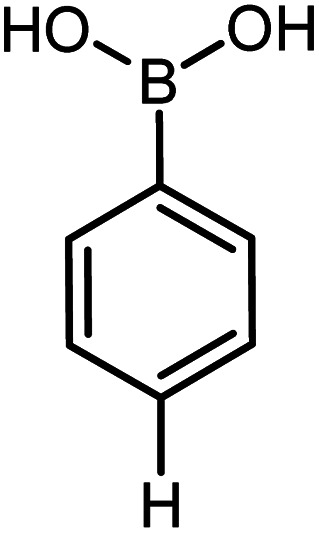
|
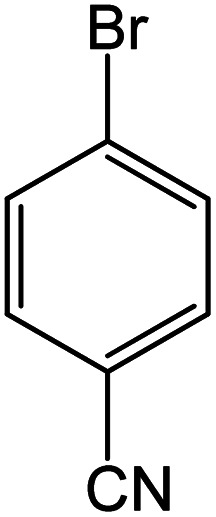
|
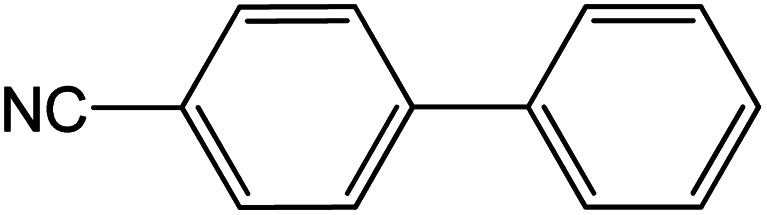
|
60 | 95 |
| 21 |
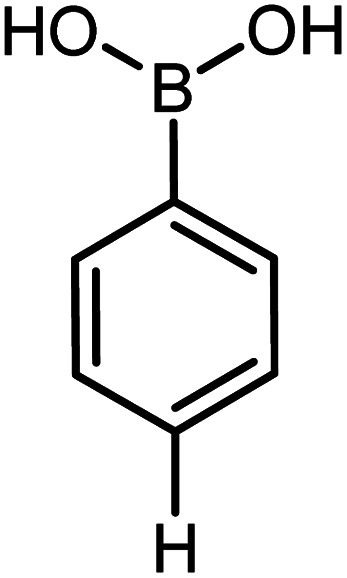
|
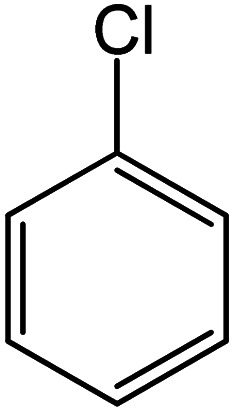
|
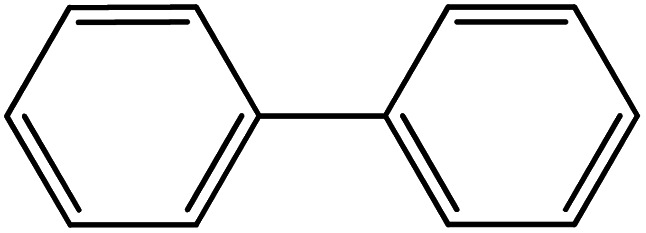
|
93 | 35 |
Conditions for the chemical reaction: 1 mmol of aryl halide, 1 mmol of aryl boronic acid, 70 °C, EtOH 78%, K2CO3.
