Figure 1.
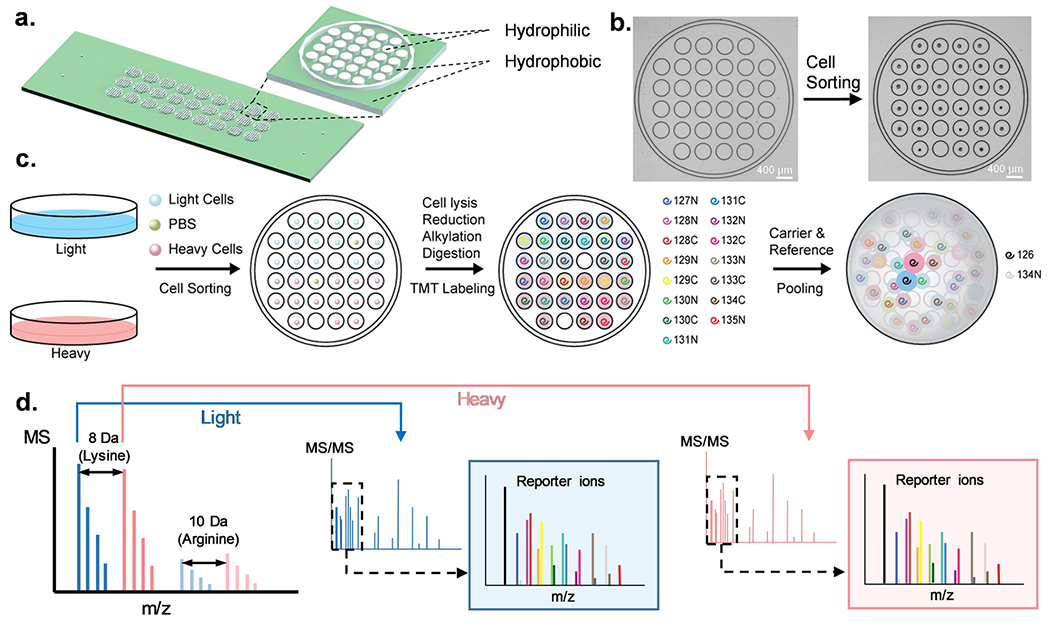
HyperSCP chip and workflow. (a) Design and structure of the hyperSCP chip. Thirty-two hydrophilic nanowells (white) are nested within a hydrophilic ring (white) that is surrounded by a hydrophobic surface (green). Twenty-seven hyperSCP sample sets can be prepared in parallel on a chip. The dimensions are shown in Figure S1. (b) Photomicrographs of a nested nanowell before and after cell sorting. Some nanowells were intentionally left empty during sorting to hold reference samples or serve as negative controls. (c) Workflow of hyperSCP sample preparation. First, the cells were cultured separately in regular media (light) or media containing 13C6 15N2 l-Lysine-2HCl and 13C6 15N4 l-Arginine-HCl (heavy). The cells and a PBS negative control sample were then sorted into each well. After cell lysis, reduction, alkylation and digestion, each single cell sample was labeled with a different TMT reagent. The carrier and reference channels were added after HA quenching, and all the peptides were pooled with 2% mobile phase B/98% mobile phase A. (d) Schematic of hyperSCP. Heavy and light precursors are identified in MS1, selected for fragmentation and analyzed by tandem MS. Reporter ions are produced for relative quantification.
