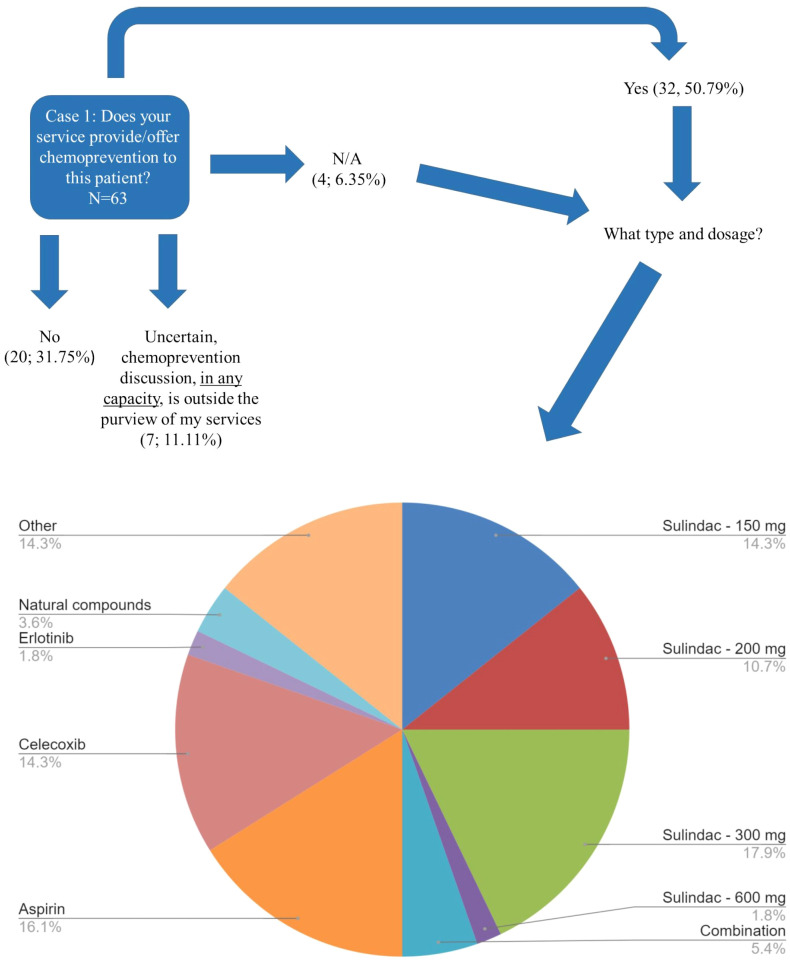
Figure 3.
Case 1 Type of Chemoprevention recommended - FAP. Responses of 63 participants regarding their recommendation regarding whether chemoprevention would be offered by their service to the patient affected with FAP described in Case 1. A subset of respondents (n=35) selected the specific type/dosage of chemoprevention offered. Respondents were allowed to select more than one option. This image depicts the 56 total selections of each management type. Of note, "Combination" is difluoromethylornithine (DFMO) plus sulindac, dosage unknown; Other: respondents could write in a response by selecting “Other”. Other could be to clarify their selection(s) and/or to provide an additional response that was not previously provided as an option. The most common write-in response was “clinical trials”. Refer to Supplementary Table 2.2S for the combinations that were selected.