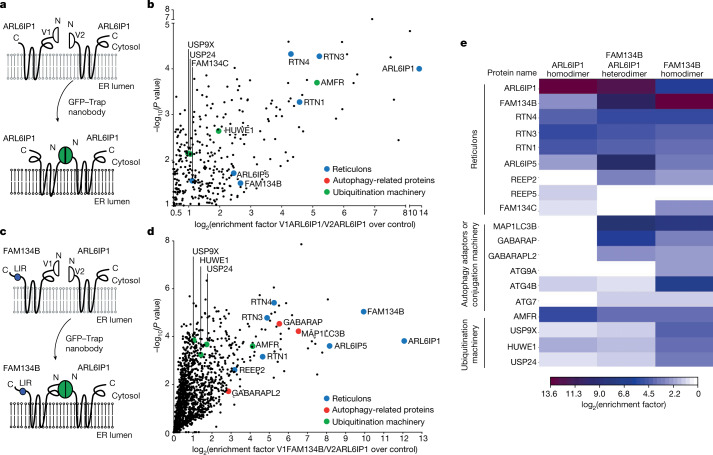
Fig. 3. ARL6IP1 supports the formation of a multi-receptor complex with FAM134B and other ER-shaping proteins.
a, ARl6IP1 N-terminally tagged with the non-fluorescent N-terminal (V1) and ARl6IP1 N-terminally tagged with the C-terminal (V2) fragment of the Venus protein exhibit fluorescence after interaction. b, Single-sided Volcano plot of the label-free interactome of ARL6IP1 homodimers revealed that FAM134B, FAM134C and other RHD-containing ER proteins (blue) and proteins of the ubiquitination machinery (green) are binding partners. Only notable hits with log2(P value) > 1 and –log10(P value) > 1.3 are labelled in colour (one-sided paired Student’s t-test with three biological replicates). c, FAM134B N-terminally tagged with the non-fluorescent N-terminal (V1) and ARL6IP1 N-terminally tagged with the C-terminal (V2) fragment of the Venus protein exhibit fluorescence after interaction. d, Single-sided volcano plot of the label-free interactome for V1-FAM134B–V2-ARL6IP1 heterodimers include autophagy-related proteins LC3B and GABARAPL2 (red) and FAM134B as known binding partners. Notable hits with log2(P value) > 1 and –log10(P value) > 1.3 are labelled in colour (one-sided paired Student’s t-test with three biological replicates). Red dots indicate autophagy-related proteins. e, Heatmap of log2 enrichment of V1-ARL6IP1–V2-ARL6IP1, V1-FAM134B–V2-ARL6IP1 or V1-FAM134B–V2-FAM134B over mock from notable hits indicated in b and d.