Figure 1.
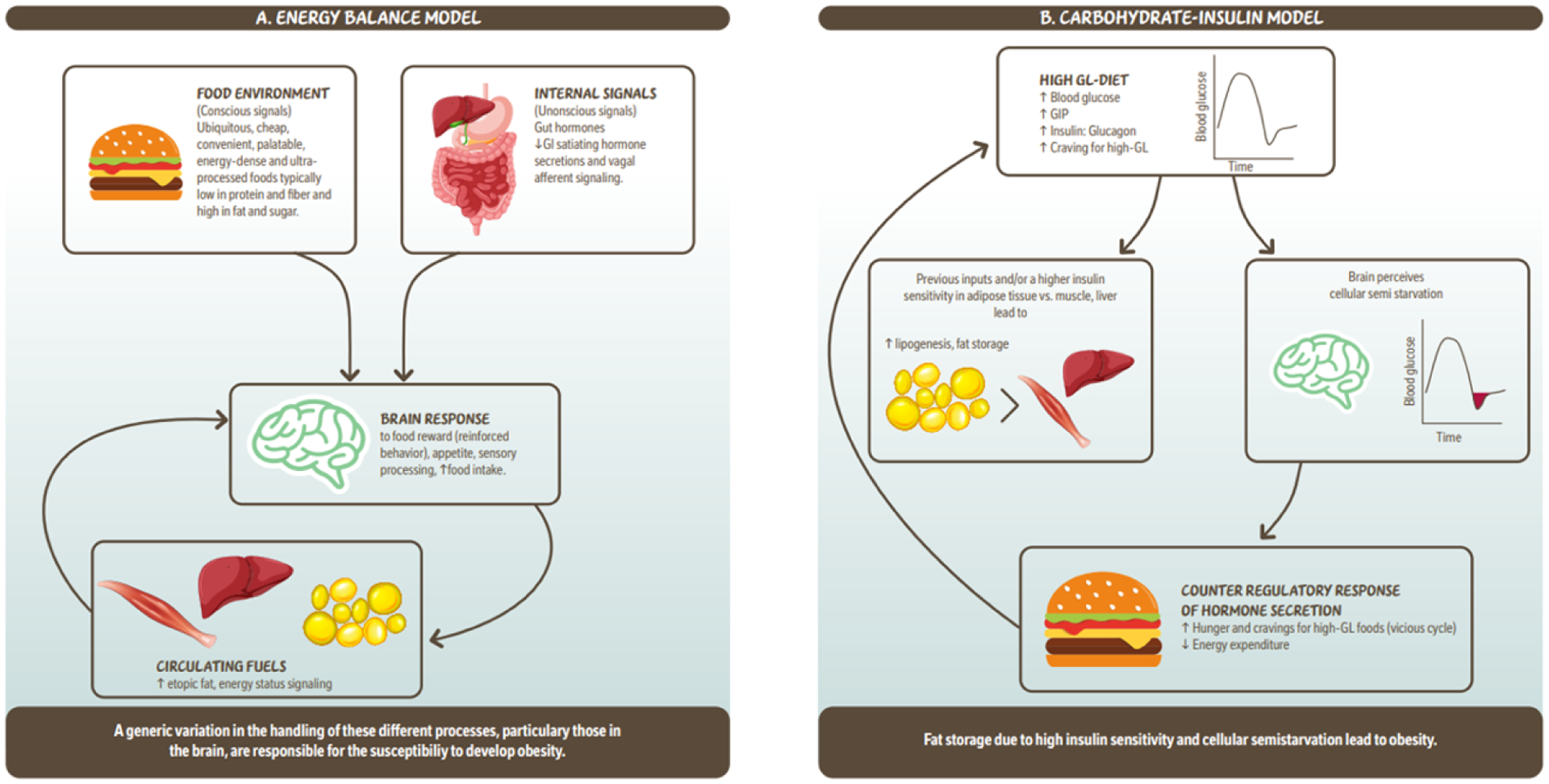
Simplified illustration of the ‘Energy balance model’ and the ‘Carbohydrate – insulin model’ of obesity. 1a. The energy balance model proposes that changes in the food environment as well as internal signals (hormones) driven by food intake, are cues to the brain which lead to increased food intake. These signals are integrated in the brain modulating behavour reinforcing of food reward, appetite which leads to more food intake and circulating fuels. Circulating fuels and signals (e.g. leptin) indicate the energy status of various organs and are sensed by the brain to control food intake. Overall this model proposes that a genetic variation in the handling of these different processes, particularly those in the brain, are responsible for the susceptibility to develop obesity. 1b. The carbohydrate- insulin model proposes that rapid absorption of glucose after consumption of a high-GL meal increases secretion of insulin to glucagon ratio, and elicits a glucose-dependent insulinotropic polypeptide (GIP)-dominant incretin response. These inputs and/or higher insulin sensitivity in adipose tissue vs. liver or muscle lead to increased lipogenesis and fat storage. The sharp insulin response and decline in blood glucose, possibly below baseline is interpreted in the brains as a state of “cellular semistarvation” and responds with a counter-regulatory hormone response and hunger and cravings for high-GL foods. Energy expenditure may also decline related to decreased fuel availability. Overall, this model proposes that fat storage due to high insulin sensitivity and cellular semistarvation lead to obesity. GI; gastrointestinal; GIP; glucose-dependent insulinotropic polypeptide; GL, glycemic load.
