Figure 3. Recombinant Chimera is reactive to human sera from SARS-CoV-2 infected patients and also from CoronaVac immunized individuals.
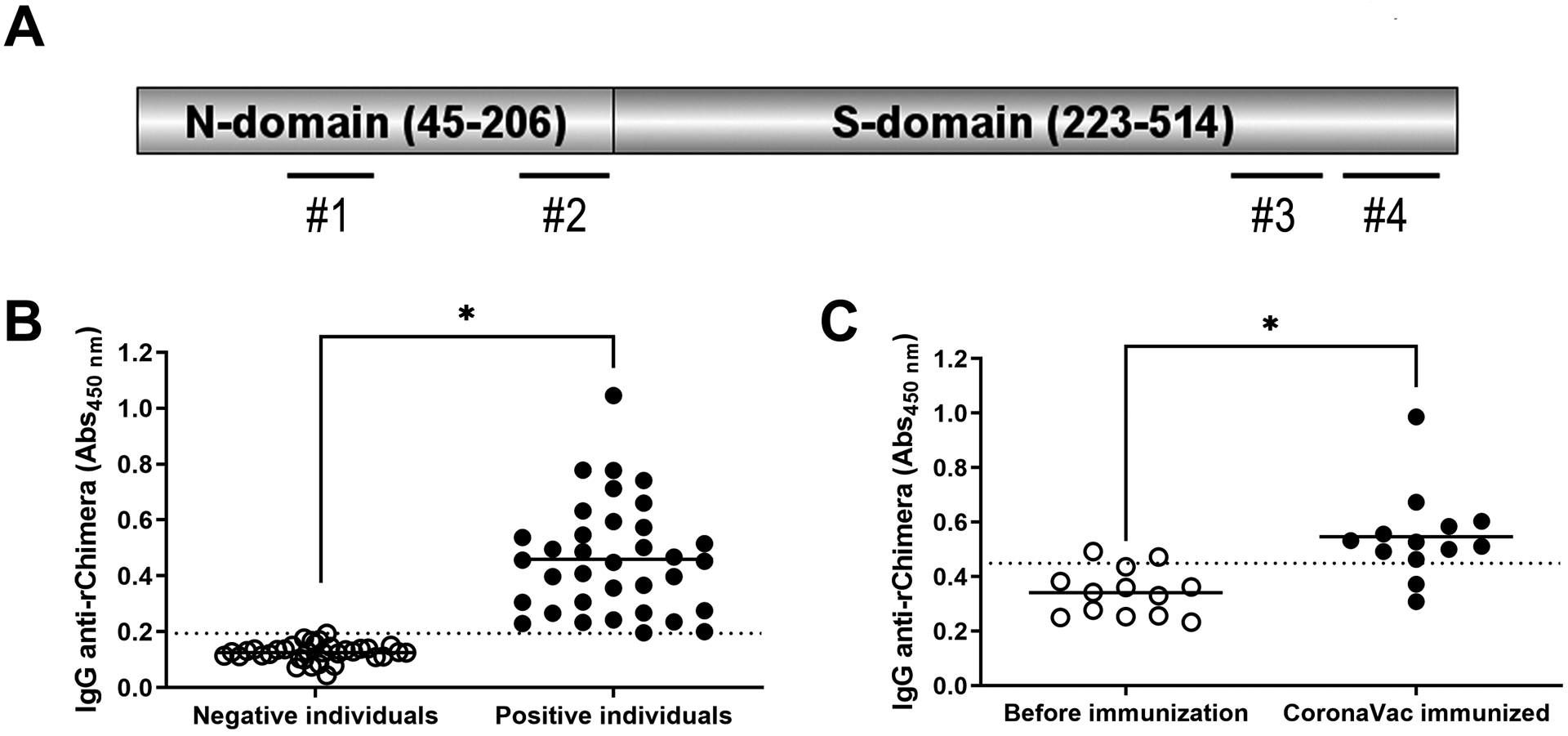
A) Schematic representation of rChimera. The N-domain is composed of 45–206 amino acids from N protein (YP_009724397.2) while the S-domain incorporates 223–514 amino acids from the S protein (YP_009724390.1). The positions of peptides #1, 2, 3 and 4 are shown by dashed lines. B) Reactivity of rChimera against human sera was assessed by ELISA. Sera from SARS-CoV-2 infected individuals (from year 2020, prior to mass vaccination and positive by molecular diagnosis) and sera from healthy individuals (from year 2015, prior to the pandemic) were used as positive and negative controls of the assay (n = 35). Sera were diluted 1:100 for the assay. C) Sera from non-SARS-CoV-2-infected individuals (negative by molecular and serological diagnosis) were assessed to rChimera’s reactivity before and after CoronaVac immunization. Samples were collected at day zero (before immunization) and three weeks after CoronaVac booster dose (n = 13). Both results are representative of total IgG antibodies against rChimera detected in the samples. Graphs are representative of two independent experiments. Statistical analysis were performed using Student’s t-test and * represents p value < 0.05. Dotted lines represent the cut-off between the samples and were obtained by ROC curve analyses.
