Abstract
Conventionally, ATP is considered to be the principal energy source in cells. However, over the last few years, a novel role for ATP as a potent extracellular signaling molecule and the principal source of extracellular pyrophosphate, the main endogenous inhibitor of vascular calcification, has emerged. A large body of evidence suggests that two principal mechanisms are involved in the initiation and progression of ectopic calcification: high phosphate concentration and pyrophosphate deficiency. Pathologic calcification of cardiovascular structures, or vascular calcification, is a feature of several genetic diseases and a common complication of chronic kidney disease, diabetes, and aging. Previous studies have shown that the loss of function of several enzymes and transporters involved in extracellular ATP/pyrophosphate metabolism is associated with vascular calcification. Therefore, pyrophosphate homeostasis should be further studied to facilitate the design of novel therapeutic approaches for ectopic calcification of cardiovascular structures, including strategies to increase pyrophosphate concentrations by targeting the ATP/pyrophosphate metabolism cycle.
Keywords: ATP, Vascular calcification, Pyrophosphate, Phosphate, Calcium
Introduction
Cardiovascular disease is the leading cause of morbidity and mortality worldwide. Pathologic cardiovascular calcification, or vascular calcification, is associated with aging and several genetic diseases and common conditions, such as diabetes and chronic kidney disease [1, 2]. Calcium and phosphate deposition, primarily in the form of hydroxyapatite, is the hallmark of vascular calcification, and this can occur in both blood vessels and cardiac valves [3, 4]. Calcified deposits can be found in heart valves and layers of the aortic wall in association with specific pathologies. For example, medial calcification, also referred to as Monckeberg’s medial sclerosis, develops in the tunica media of the aorta, in association with the elastic lamina, whereas intimal calcification occurs in atherosclerotic lesions [5].
Classically, ATP is considered to be the principal energy source in cells. However, over the last few years, a novel role for ATP as a potent extracellular signaling molecule and source of extracellular pyrophosphate, the principal endogenous inhibitor of vascular calcification, has emerged. Several ectoenzymes use extracellular ATP for this purpose [6, 7], including ectonucleotide pyrophosphatase/phosphodiesterase (eNPP) and ectonucleoside triphosphate diphosphohydrolase (eNTPD). In addition, previous studies have shown that ATP plays a key role as a direct inhibitor of vascular calcification [8, 9].
Phosphate and vascular calcification
High inorganic phosphate concentration has emerged as a key risk factor for vascular calcification in the general population and in patients with chronic kidney disease [3]. For example, high-serum phosphate concentration is a typical abnormality in patients undergoing long-term hemodialysis if they are not administered phosphate binders or do not restrict their dietary phosphate intake, and this contributes to their high morbidity and mortality rates [10]. A number of factors play important roles in phosphate homeostasis by influencing phosphate turnover in the kidney, intestine, and bone. Greater absorption or lower excretion of phosphate increases serum phosphate concentration, which is associated with the deposition of calcium and phosphate in blood vessels [3]. The dysregulation of phosphate homeostasis is a feature of several diseases, including hyperparathyroidism, hyper- and hypovitaminosis D, chronic renal disease, osteoporosis, and diabetes mellitus [3].
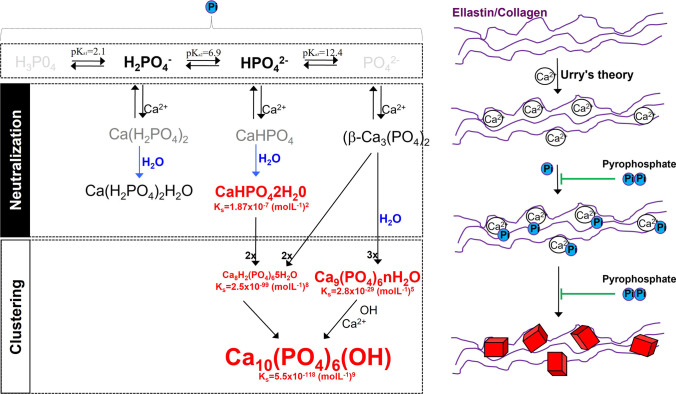
In biological systems, phosphate exists in four forms (see Fig. 1) that are classified on the basis of the triprotic equilibrium: trihydrogen phosphate (H3PO4), dihydrogen phosphate ion (H2PO4−), hydrogen phosphate ion (HPO42−), and phosphate ion (PO43−). In the presence of calcium ions, various phosphate-calcium salts are produced by the charge neutralization of these phosphate ions, including anhydrous monocalcium phosphate (Ca(H2PO4)2), anhydrous dicalcium phosphate (CaHPO4), and β-tricalcium phosphate (β-Ca3(PO4)2) [11]. The anhydrous calcium-phosphate salts can be hydrated to form monocalcium phosphate monohydrate (Ca(H2PO4)2.H2O) and dicalcium phosphate dihydrate (CaHPO4.2H2O; also called Brushite), respectively [12]. The ultimate product of the reaction between calcium and phosphate is crystalline hydroxyapatite (Ca10(PO4)6(OH)), which is the principal component of bone and calcified tissues [13], but there are also two precursors of hydroxyapatite: amorphous calcium phosphate (Ca9(PO4)6.nH2O) and octocalcium phosphate (Ca8H2(PO4)6.5H2O), which, like Brushite, are often found in calcified tissues [11].
Fig. 1.
Schematic representation of calcification. Inorganic phosphate (Pi) exists in several forms including trihydrogen phosphate (H3P04), dihydrogen phosphate ion (H2PO4−), hydrogen phosphate ion (HPO42−), and phosphate ion (PO42−). Phosphate charge is neutralized with calcium forming several calcium-phosphate salts including, anhydrous monocalcium phosphate (Ca(H2PO4)2), anhydrous dicalcium phosphate (CaHPO4), β-tricalcium phosphate (β-Ca3(PO4)2), monocalcium phosphate monohydrate (Ca(H2PO4)2H2O), and dicalcium phosphate dihydrate (CaHPO42H20; also called Brushite). The final product of the calcium and phosphate reaction is crystalline hydroxyapatite (Ca10(PO4)6(OH)), the main component of bone and calcified tissues and two of its precursors, amorphous calcium phosphate (Ca9(PO4)6nH2O), and octocalcium phosphate (Ca8H2(PO4)65H2O). Pyrophosphate directly inhibits the formation and growth of these phosphate-calcium crystals. Ks, solubility product constant
These calcium-phosphate salts are deposited on extracellular matrix proteins, such as elastin and collagen, in vitro and in vivo [5, 14, 15]. According to the charge neutralization theory of calcification [5], the high glycine content of these matrix proteins favors the formation of beta-turns that can interact with calcium ions (see Fig. 1). In bone and connective tissue, these salts are predominantly deposited on type I collagen and elastic fibers, respectively.
In vitro experiments have revealed that calcium-phosphate crystals are deposited in aortic smooth muscle cells following their incubation in a medium containing a high phosphate concentration [16]. There are two principal potential consequences for aortic smooth muscle cells that undergo phosphate-induced vascular calcification. One is apoptosis-dependent matrix mineralization, which has been identified in cultured human aortic smooth muscle cells and arteries from pediatric patients undergoing dialysis [17, 18]. The other is a profound transition to a bone-forming phenotype, which results in the loss of aortic smooth muscle cell markers, such as smooth muscle α-actin (SM22α), and the expression of osteochondrogenic markers, such as runt-related transcription factor (Runx)2/Cbfa1 and bone morphogenic protein (BMP)-2[19]. Recent studies have demonstrated that calcium-phosphate deposits can induce a transition to a bone-forming phenotype, as well as apoptosis, in aortic smooth muscle cells, suggesting that the mechanisms described could be a result of ectopic calcification [14, 20].
Pyrophosphate and vascular calcification
Extracellular fluids in vertebrates are supersaturated with phosphate and calcium, which results in a tendency toward spontaneous calcium-phosphate precipitation (see Fig. 1). There is a range of endogenous low- and high-molecular-weight precipitation inhibitors in extracellular fluids that are essential for survival, including small and medium-sized proteins, such as matrix Gla proteins, fetuin A, and osteopontin, and small molecules, such as pyrophosphate [4].
Extracellular pyrophosphate is a potent endogenous inhibitor of hydroxyapatite crystal formation and growth in vitro and in vivo [21]. Recent studies have shown that the endogenous production of pyrophosphate can prevent vascular calcification [22]. However, although pyrophosphate can prevent calcification when phosphate is present at normal concentrations [23], it is insufficient to prevent hydroxyapatite crystal formation in the presence of hyperphosphatemia. Lomashvili et al. [24] demonstrated a more significant role of systemic pyrophosphate (plasma pyrophosphate concentration), than local pyrophosphate production for the prevention of vascular calcification.
Low pyrophosphate concentration is associated with ectopic calcification [25]. However, circulating pyrophosphate concentration does not correlate, alone, with the severity of calcification. Patients undergoing hemodialysis have low plasma pyrophosphate concentrations [22, 26], as do patients with pseudoxanthoma elasticum [27]. Moreover, mouse models of progeria [9, 28] and pseudoxanthoma elasticum (PXE) [29], which are both characterized by calcification, show low plasma pyrophosphate concentrations. Daily injections of pyrophosphate prevent calcification in both of these models [28, 29], and exogenous pyrophosphate administration has been shown to prevent uremic calcification in other rodent models [30, 31]. Finally, other studies have shown that the oral administration of pyrophosphate prevents calcification in mouse models of generalized arterial calcification of infancy and PXE [32, 33].
Extracellular ATP/pyrophosphate metabolism cycle and genetic disorders
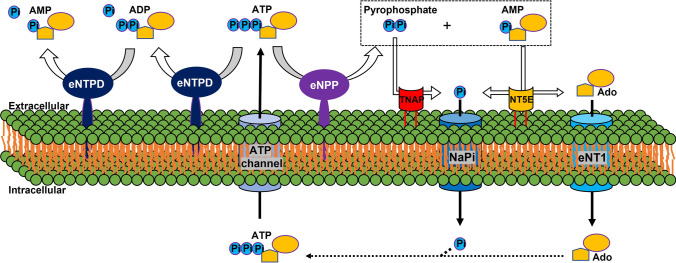
The extracellular ATP metabolism cycle begins with the transport of ATP to the extracellular space (see Fig. 2). Cellular ATP release occurs through exocytotic mechanisms and by membrane protein transport, including via connexin hemichannels, pannexin, and multidrug resistance-associated protein 6 (MPR6, also known as ATP-binding cassette sub-family C member 6 (ABCC6)) [34]. Notably, ABCC6 facilitates ATP excretion in the liver and is responsible for 60–70% of all the pyrophosphate present in plasma [27, 35]. Mutations in ABCC6 can cause PXE, a heritable connective tissue disorder that is characterized by testicular microlithiasis and the calcification of the elastic fibers of skin arteries and Bruch’s membrane [36, 37]. Loss of function ABCC6 mutations result in lower extracellular pyrophosphate concentrations. However, the function of ABCC6 and the pathogenesis of PXE have not been fully characterized.
Fig. 2.
Schematic representation of the ectoenzymes and transporters involved in the extracellular ATP/pyrophosphate metabolism cycle. eNPP, ectonucleotide pyrophosphatase phosphodiesterase; eNTPD, ectonucleoside triphosphate diphosphohydrolase; ATP, adenosine-5’-triphosphate; ADP, adenosine-5’-diphosphate; AMP, adenosine-5’-monophosphate; Ado, adenosine; TNAP, tissue nonspecific alkaline phosphatase; ANK, the progressive ankylosis protein; NaPi, sodium-phosphate cotransporters; NT5E, ecto-5′nucletotidase; eNT1, equilibrative nucleoside transporter 1. ATP channel includes ABCC6
Extracellular ATP is hydrolyzed by eNPP (ectonucleotide pyrophosphatase/phosphodiesterase) family to generate extracellular AMP and pyrophosphate (see Fig. 2) [6, 38]. eNPP1 is the principal enzyme responsible for the production of extracellular pyrophosphate in vascular smooth muscle cells and the aorta[15, 38], and mutations in the gene encoding this enzyme can result in generalized arterial calcification of infancy, which is characterized by the calcification of the internal elastic laminae of large- and medium-sized arteries (see Table 1) [39, 40]. Moreover, eNPP1-null mice show widespread ectopic arterial calcification [41].
Table 1.
Genetic disease involved in extracellular ATP/pyrophosphate metabolism associated with ectopic calcification
| Protein affected | Role | Genetic disease | Ectopic calcification | Year [ref.] |
|---|---|---|---|---|
| ABCC6 | Facilitates ATP transport to extracellular milieu | Pseudoxanthoma elasticum | Elastic fibers in skin, arteries, and Bruch’s membrane | 2000 [36, 37] |
| ANK | ? | Craniometaphyseal dysplasia | Craniofacial bones | 2001 [62] |
| ANK | ? | Chondrocalcinosis | Articular cartilage | 2002 [72] |
| eNPP1 | Pyrophosphate synthesis | Generalized arterial calcification in infancy | Medial arterial | 2003 [39] |
| NT5E | Hydrolysis of AMP | Medial arterial and periarticular | 2011 [42] | |
| Pit-2 | Phosphate transporter | Familial idiopathic basal ganglia calcification (type 1) | Basal ganglia and cortex | 2012 [58] |
| ENT1 | Ado transporter | Idiopathic skeletal hyperostosis | Spinal tissues | 2013 [51] |
Notably, a direct competitor of eNPP for the substrate is the ectonucleoside triphosphate diphosphohydrolase family (eNTPD, see Fig. 2), which comprises well-known ectoenzymes in the purinergic signaling pathway. Members of the eNTPD family, including eNTPD1 (CD39) hydrolyze ATP and ADP, with differing preferences for the specific type of nucleotide [9, 10]. eNTPD1 is the principal ectoenzyme in the rat aorta, where it hydrolyzes 90% of the ATP and releases a small amount of ADP because of its high affinity for ADP (with an ATP/ADP ratio of 1:0.8) [8, 15, 38]. The largest body of evidence of a direct link to the regulation of signaling through purine receptors is available for eNTPD1, but this has not been linked to ectopic calcification. However, recent studies have indirectly linked eNTPD1 with vascular calcification [9, 15, 28]. Smooth muscle cells derived from the aortas of a new mouse model of Hutchinson-Gilford progeria syndrome, which is characterized by excessive vascular calcification, show high expression and activity of eNTPD1 [28]. These data suggest that eNTPD1 might limit the availability of ATP for the production of pyrophosphate by eNPP1 [15]. Moreover, the inhibition of eNTPD prevents vascular calcification in progeroid mice [9]. Therefore, the expression and regulation of this enzyme in the context of ectopic calcification should be further explored.
Another ectoenzyme involved in extracellular pyrophosphate metabolism is the membrane-bound ecto-5′ nucleotidase (NT5E, also referred to as CD73). This enzyme binds AMP molecules and converts them to adenosine and phosphate (see Fig. 2) [6, 7]. Mutations in NT5E can induce calcification of the tunica media of the arteries of the lower extremities and periarticular calcification in both humans and mice (see Table 1) [40, 42, 43]. Notably, NT5E-null mice have high plasma phosphate concentrations and low plasma pyrophosphate concentrations [43].
Extracellular pyrophosphate is principally degraded to phosphate by tissue nonspecific alkaline phosphatase (TNAP) in extracellular fluids (see Fig. 2) [6, 38]. However, other enzymes can also hydrolyze extracellular pyrophosphate in smaller amounts, including eNPP3 [38]. The overexpression of TNAP is sufficient to induce medial vascular calcification in aortic rings ex vivo, and the addition of alkaline phosphatase to the culture media is sufficient to cause matrix calcification [38]. In vivo overexpression of TNAP increases skeletal mineralization [44]. Moreover, TNAP activity is high in several models of medial vascular calcification, including rats with uremia and a mouse model of Hutchinson-Gilford progeria syndrome [26, 28, 45]. Notably, induced pluripotent stem cell-derived mesenchymal stromal cells from patients with arterial calcification owing to NT5E deficiency show higher TNAP activity than cells derived from healthy individuals [46, 47]. Previous studies have shown that phosphatase inhibitors prevent vascular smooth muscle calcification in vitro [48], and a selective and orally bioavailable TNAP inhibitor attenuates the development and progression of calcification in ABCC6-null mice [49], but not in ENPP1 mutant mice [50].
Adenosine and phosphate must be recovered from the extracellular space to regenerate ATP in mitochondria or through another metabolic pathway (see Fig. 2). The results of a study of equilibrative nucleoside transporter 1 (ENT1, SLC29A1) deficiency in mice may help explain the basis of diffuse idiopathic skeletal hyperostosis in humans, which is characterized by the ectopic calcification of spinal tissues (see Table 1) [51]. In this study, it was shown that expression of eNPP1, the progressive ankylosis (ANK) gene, and TNAP was significantly lower in the intervertebral disks of ENT1-null mice than in those of wild-type mice.
Cellular phosphate levels are controlled by sodium-phosphate cotransporters (NPTs) [52]. Two main families of NPT (also known as NaPi) have been identified: type II (SLC34) and type III (SLC20) [52]. These display high (Km ≤ 0.1 mmol/L) but differing affinities for H2PO4− and HPO42− ions [53]. The type II sodium-phosphate cotransporter family (also called NaPi-II) comprises three members that are expressed in the small intestine (NaPi-IIb) and kidney (NaPi-IIa and NaPi-IIc), two important sites for phosphate homeostasis. Type III sodium-phosphate cotransporters include Pit-1 and Pit-2 [54], which are ubiquitously expressed and mediate the movement of phosphate ions across the cell membrane. The expression of Pit1 mRNA is highest in vascular smooth muscle cells [55, 56], but it is also expressed in osteoblasts and bone marrow. However, deletion of Pit-1 in vascular smooth muscle cells in mice does not induce aortic calcification, because of compensation by Pit-2[57]. The expression of Pit-2 is highest in vascular smooth muscle cells, liver, heart, and brain. Recently, an association between Pit-2 loss of function and type I familial idiopathic basal ganglial calcification in humans has been reported [58], and the global knockout of Pit-2 in mice is associated with calcification in the thalamus, basal ganglia, and cortex [59], suggesting that low Pit-2 expression on its own can cause brain calcification.
Finally, previous studies have suggested that ANK plays an important role in ATP/pyrophosphate metabolism [60, 61]. Mutation of the ANK gene causes a severe form of generalized joint calcification, and arthritis, associated with excessive hydroxyapatite formation, characterizes Ank−/− mice. ANK-mutant cells have low extracellular pyrophosphate concentrations, and ANK overexpression in cultured cells and tissues increases extracellular pyrophosphate concentration [60]. Several helix prediction programs have shown that the ANK protein has 7–12 membrane-spanning helices and a central channel [60, 62, 63]. Therefore, ANK may regulate pyrophosphate transport from the cytoplasm to the extracellular milieu [60, 62, 64]. However, recent studies have shown that ANK may be a channel or regulator of adjacent channels that mediate the extracellular transport of ATP [61, 65, 66]. For example, several studies have shown that inhibition of ANK using probenecid attenuates extracellular ATP accumulation [65, 66]. In contrast, HEK293 cells overexpressing wild-type ANKH cause greater accumulation of both pyrophosphate and ATP in the culture medium [61], as well as increases in the concentrations of citrate, malate, succinate, and several nucleoside monophosphates. However, the direct effects of ANK that cause pyrophosphate/ATP release have not been demonstrated.
Notably, several authors have suggested that ANK may play a key role in the regulation of the complex interplay of proteins that influences pyrophosphate and phosphate concentrations (pyrophosphate/phosphate ratio) by controlling ATP transport through specific channels and regulating phosphate transport and the activities of eNPP1 and TNAP [67–71]. For example, the transfection of eNPP1 into osteoblasts increases extracellular pyrophosphate concentration only when wild-type ANK is present [68], M48T mutation of ANK interferes with the interaction between ANK and the sodium/phosphate cotransporter Pit-1 [70], and the overexpression of wild-type ANK protein results in the downregulation of TNAP in chondrogenic cells [69]. In support of this notion, mutations in sequences that encode the channel core of ANK cause craniometaphyseal dysplasia [62, 63]. This rare skeletal condition is characterized by abnormal bone formation, with a higher density of the craniofacial bones and abnormal modeling of the metaphyses of long bones, and is associated with lower extracellular pyrophosphate concentration. In contrast, mutations in sequences that encode the N- and C-termini of the ANK protein cause chondrocalcinosis [72]. This articular cartilage disorder is radiographically characterized by the deposition of calcium pyrophosphate dihydrate crystals in joints and is associated with a high extracellular pyrophosphate concentration. Therefore, the role of ANK in extracellular ATP/pyrophosphate metabolism should be studied in more depth.
Summary and perspective
A large body of evidence suggests that two principal mechanisms are involved in the initiation and progression of ectopic calcification: high phosphate concentration and pyrophosphate deficiency. The circulating pyrophosphate concentration does not correlate, alone, with the severity of calcification, which implies that phosphate and pyrophosphate concentrations need to be appropriately balanced [73]. Therefore, phosphate and pyrophosphate homeostasis should be further studied to facilitate the design of novel therapeutic approaches for ectopic calcification of cardiovascular structures, including strategies to increase pyrophosphate concentrations by targeting the ATP/pyrophosphate metabolism cycle.
Ricardo Villa-Bellosta
holds a PhD (2010) by the University of Zaragoza, Spain. His doctoral thesis got the Spanish Royal Academy of Doctors Award and Extraordinary doctoral Award. He joined (2021) the Center for Research in Molecular Medicine and Chronic Disease (CiMUS) in Santiago de Compostela University, as group leader of the “Metabolic Homeostasis and Vascular Calcification” laboratory. His laboratory is focused on the role of phosphate and pyrophosphate homeostasis in vascular calcification in progeria, aging, diabetes, chronic kidney disease and several genetic disorders. 
Funding
RV-B is a Senior Postdoctoral “Ramon y Cajal” Researcher (RYC2019-027920-I) supported by grants from the Spanish Ministerio de Ciencia e Innovacion (PID2020-113603RB-I00) and Spanish Society of Nephrology (SEN21-3315).
Data availability
Data not available to be shared.
Declarations
Ethical approval
Ethical approval is not applicable to this article.
Informed consent
There are no human subjects in this article and informed consent is not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen NX, Moe SM. Pathophysiology of vascular calcification. Curr Osteoporos Rep. 2015;13(6):372–380. doi: 10.1007/s11914-015-0293-9. [DOI] [PubMed] [Google Scholar]
- 2.Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv. 2014;83(6):E212–220. doi: 10.1002/ccd.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 2011;109(6):697–711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutsch F, Nitschke Y, Terkeltaub R. Genetics in arterial calcification: pieces of a puzzle and cogs in a wheel. Circ Res. 2011;109(5):578–592. doi: 10.1161/CIRCRESAHA.111.247965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urry DW. Neutral sites for calcium ion binding to elastin and collagen: a charge neutralization theory for calcification and its relationship to atherosclerosis. Proc Natl Acad Sci U S A. 1971;68(4):810–814. doi: 10.1073/pnas.68.4.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8(3):437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(4–5):299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 8.Villa-Bellosta R, Sorribas V. Prevention of vascular calcification by polyphosphates and nucleotides- role of ATP. Circ J. 2013;77(8):2145–2151. doi: 10.1253/circj.CJ-13-0016. [DOI] [PubMed] [Google Scholar]
- 9.Villa-Bellosta R. ATP-based therapy prevents vascular calcification and extends longevity in a mouse model of Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2019;116(47):23698–23704. doi: 10.1073/pnas.1910972116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 11.LeGeros RZ. Formation and transformation of calcium phosphates: relevance to vascular calcification. Z Kardiol. 2001;90(Suppl 3):116–124. doi: 10.1007/s003920170032. [DOI] [PubMed] [Google Scholar]
- 12.Johnsson MS, Nancollas GH. The role of brushite and octacalcium phosphate in apatite formation. Crit Rev Oral Biol Med Off Publ Am Assoc Oral Biol. 1992;3(1–2):61–82. doi: 10.1177/10454411920030010601. [DOI] [PubMed] [Google Scholar]
- 13.Kay MI, Young RA, Posner AS. Crystal structure of hydroxyapatite. Nature. 1964;204:1050–1052. doi: 10.1038/2041050a0. [DOI] [PubMed] [Google Scholar]
- 14.Villa-Bellosta R, Millan A, Sorribas V. Role of calcium-phosphate deposition in vascular smooth muscle cell calcification. Am J Physiol Cell Physiol. 2011;300(1):C210–220. doi: 10.1152/ajpcell.00229.2010. [DOI] [PubMed] [Google Scholar]
- 15.Villa-Bellosta R. Synthesis of extracellular pyrophosphate increases in vascular smooth muscle cells during phosphate-induced calcification. Arterioscler Thromb Vasc Biol. 2018;38(9):2137–2147. doi: 10.1161/ATVBAHA.118.311444. [DOI] [PubMed] [Google Scholar]
- 16.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87(7):E10–17. doi: 10.1161/01.RES.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 17.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87(11):1055–1062. doi: 10.1161/01.RES.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 18.Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118(17):1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 19.Speer MY, Li X, Hiremath PG, Giachelli CM. Runx2/Cbfa1, but not loss of myocardin, is required for smooth muscle cell lineage reprogramming toward osteochondrogenesis. J Cell Biochem. 2010;110(4):935–947. doi: 10.1002/jcb.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sage AP, Lu J, Tintut Y, Demer LL. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 2011;79(4):414–422. doi: 10.1038/ki.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schibler D, Russell RG, Fleisch H. Inhibition by pyrophosphate and polyphosphate of aortic calcification induced by vitamin D3 in rats. Clin Sci. 1968;35(2):363–372. [PubMed] [Google Scholar]
- 22.Lomashvili KA, Khawandi W, O’Neill WC. Reduced plasma pyrophosphate levels in hemodialysis patients. J Am Soc Nephrol. 2005;16(8):2495–2500. doi: 10.1681/ASN.2004080694. [DOI] [PubMed] [Google Scholar]
- 23.Villa-Bellosta R, Sorribas V. Calcium phosphate deposition with normal phosphate concentration. Role of pyrophosphate. Circ J. 2011;75(11):2705–10. doi: 10.1253/circj.CJ-11-0477. [DOI] [PubMed] [Google Scholar]
- 24.Lomashvili KA, Narisawa S, Millán JL, O’Neill WC. Vascular calcification is dependent on plasma levels of pyrophosphate. Kidney Int. 2014;85(6):1351–1356. doi: 10.1038/ki.2013.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villa-Bellosta R, O’Neill WC. Pyrophosphate deficiency in vascular calcification. Kidney Int. 2018;93(6):1293–1297. doi: 10.1016/j.kint.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 26.Azpiazu D, González-Parra E, Egido J, Villa-Bellosta R. Hydrolysis of extracellular pyrophosphate increases in post-hemodialysis plasma. Sci Rep. 2018;8(1):11089. doi: 10.1038/s41598-018-29432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansen RS, Duijst S, Mahakena S, Sommer D, Szeri F, Váradi A, et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler Thromb Vasc Biol. 2014;34(9):1985–1989. doi: 10.1161/ATVBAHA.114.304017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villa-Bellosta R, Rivera-Torres J, Osorio FG, Acín-Pérez R, Enriquez JA, López-Otín C, et al. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson-Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation. 2013;127(24):2442–2451. doi: 10.1161/CIRCULATIONAHA.112.000571. [DOI] [PubMed] [Google Scholar]
- 29.Pomozi V, Brampton C, van de Wetering K, Zoll J, Calio B, Pham K, et al. Pyrophosphate supplementation prevents chronic and acute calcification in ABCC6-deficient mice. Am J Pathol. 2017;187(6):1258–1272. doi: 10.1016/j.ajpath.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Neill WC, Lomashvili KA, Malluche HH, Faugere M-C, Riser BL. Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int. 2011;79(5):512–517. doi: 10.1038/ki.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riser BL, Barreto FC, Rezg R, Valaitis PW, Cook CS, White JA, et al. Daily peritoneal administration of sodium pyrophosphate in a dialysis solution prevents the development of vascular calcification in a mouse model of uraemia. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2011;26(10):3349–3357. doi: 10.1093/ndt/gfr039. [DOI] [PubMed] [Google Scholar]
- 32.Dedinszki D, Szeri F, Kozák E, Pomozi V, Tőkési N, Mezei TR, et al. Oral administration of pyrophosphate inhibits connective tissue calcification. EMBO Mol Med. 2017;9(11):1463–1470. doi: 10.15252/emmm.201707532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozák E, Fülöp K, Tőkési N, Rao N, Li Q, Terry SF, et al. Oral supplementation of inorganic pyrophosphate in pseudoxanthoma elasticum. Exp Dermatol. 2022;31(4):548–555. doi: 10.1111/exd.14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lohman AW, Billaud M, Isakson BE. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res. 2012;95(3):269–280. doi: 10.1093/cvr/cvs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jansen RS, Küçükosmanoglu A, de Haas M, Sapthu S, Otero JA, Hegman IEM, et al. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Natl Acad Sci U S A. 2013;110(50):20206–20211. doi: 10.1073/pnas.1319582110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet. 2000;25(2):228–231. doi: 10.1038/76109. [DOI] [PubMed] [Google Scholar]
- 37.Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet. 2000;25(2):223–227. doi: 10.1038/76102. [DOI] [PubMed] [Google Scholar]
- 38.Villa-Bellosta R, Wang X, Millán JL, Dubyak GR, O’Neill WC. Extracellular pyrophosphate metabolism and calcification in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2011;301(1):H61–68. doi: 10.1152/ajpheart.01020.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Höhne W, et al. Mutations in ENPP1 are associated with «idiopathic» infantile arterial calcification. Nat Genet. 2003;34(4):379–381. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- 40.Rutsch F, Buers I, Nitschke Y. Hereditary disorders of cardiovascular calcification. Arterioscler Thromb Vasc Biol. 2021;41(1):35–47. doi: 10.1161/ATVBAHA.120.315577. [DOI] [PubMed] [Google Scholar]
- 41.Johnson K, Polewski M, van Etten D, Terkeltaub R. Chondrogenesis mediated by PPi depletion promotes spontaneous aortic calcification in NPP1-/- mice. Arterioscler Thromb Vasc Biol. 2005;25(4):686–691. doi: 10.1161/01.ATV.0000154774.71187.f0. [DOI] [PubMed] [Google Scholar]
- 42.St Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, et al. NT5E mutations and arterial calcifications. N Engl J Med. 2011;364(5):432–442. doi: 10.1056/NEJMoa0912923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Q, Price TP, Sundberg JP, Uitto J. Juxta-articular joint-capsule mineralization in CD73 deficient mice: similarities to patients with NT5E mutations. Cell Cycle Georget Tex. 2014;13(16):2609–2615. doi: 10.4161/15384101.2014.943567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narisawa S, Yadav MC, Millán JL. In vivo overexpression of tissue-nonspecific alkaline phosphatase increases skeletal mineralization and affects the phosphorylation status of osteopontin. J Bone Miner Res Off J Am Soc Bone Miner Res. 2013;28(7):1587–1598. doi: 10.1002/jbmr.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lomashvili KA, Garg P, Narisawa S, Millan JL, O’Neill WC. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int. 2008;73(9):1024–1030. doi: 10.1038/ki.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin H, St Hilaire C, Huang Y, Yang D, Dmitrieva NI, Negro A, et al. Increased activity of TNAP compensates for reduced adenosine production and promotes ectopic calcification in the genetic disease ACDC. Sci Signal. 2016;9(458):ra121. doi: 10.1126/scisignal.aaf9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moorhead WJ, Chu CC, Cuevas RA, Callahan J, Wong R, Regan C, et al. Dysregulation of FOXO1 (Forkhead Box O1 Protein) drives calcification in arterial calcification due to deficiency of CD73 and is present in peripheral artery disease. Arterioscler Thromb Vasc Biol. 2020;40(7):1680–1694. doi: 10.1161/ATVBAHA.119.313765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narisawa S, Harmey D, Yadav MC, O’Neill WC, Hoylaerts MF, Millán JL. Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J Bone Miner Res Off J Am Soc Bone Miner Res. 2007;22(11):1700–1710. doi: 10.1359/jbmr.070714. [DOI] [PubMed] [Google Scholar]
- 49.Ziegler SG, Ferreira CR, MacFarlane EG, Riddle RC, Tomlinson RE, Chew EY, et al. Ectopic calcification in pseudoxanthoma elasticum responds to inhibition of tissue-nonspecific alkaline phosphatase. Sci Transl Med. 2017;9(393):eaal1669. doi: 10.1126/scitranslmed.aal1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Q, Huang J, Pinkerton AB, Millan JL, van Zelst BD, Levine MA, et al. Inhibition of tissue-nonspecific alkaline phosphatase attenuates ectopic mineralization in the Abcc6-/- mouse model of PXE but not in the Enpp1 mutant mouse models of GACI. J Invest Dermatol. 2019;139(2):360–368. doi: 10.1016/j.jid.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warraich S, Bone DBJ, Quinonez D, Ii H, Choi D-S, Holdsworth DW, et al. Loss of equilibrative nucleoside transporter 1 in mice leads to progressive ectopic mineralization of spinal tissues resembling diffuse idiopathic skeletal hyperostosis in humans. J Bone Miner Res Off J Am Soc Bone Miner Res. 2013;28(5):1135–1149. doi: 10.1002/jbmr.1826. [DOI] [PubMed] [Google Scholar]
- 52.Forster IC, Hernando N, Biber J, Murer H. Phosphate transporters of the SLC20 and SLC34 families. Mol Aspects Med. 2013;34(2–3):386–395. doi: 10.1016/j.mam.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Villa-Bellosta R, Sorribas V. Role of rat sodium/phosphate cotransporters in the cell membrane transport of arsenate. Toxicol Appl Pharmacol. 2008;232(1):125–134. doi: 10.1016/j.taap.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 54.Wagner CA, Hernando N, Forster IC, Biber J. The SLC34 family of sodium-dependent phosphate transporters. Pflugers Arch. 2014;466(1):139–153. doi: 10.1007/s00424-013-1418-6. [DOI] [PubMed] [Google Scholar]
- 55.Villa-Bellosta R, Bogaert YE, Levi M, Sorribas V. Characterization of phosphate transport in rat vascular smooth muscle cells: implications for vascular calcification. Arterioscler Thromb Vasc Biol. 2007;27(5):1030–1036. doi: 10.1161/ATVBAHA.106.132266. [DOI] [PubMed] [Google Scholar]
- 56.Li X, Yang H-Y, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98(7):905–912. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 57.Crouthamel MH, Lau WL, Leaf EM, Chavkin NW, Wallingford MC, Peterson DF, et al. Sodium-dependent phosphate cotransporters and phosphate-induced calcification of vascular smooth muscle cells: redundant roles for PiT-1 and PiT-2. Arterioscler Thromb Vasc Biol. 2013;33(11):2625–2632. doi: 10.1161/ATVBAHA.113.302249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang C, Li Y, Shi L, Ren J, Patti M, Wang T, et al. Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat Genet. 2012;44(3):254–256. doi: 10.1038/ng.1077. [DOI] [PubMed] [Google Scholar]
- 59.Jensen N, Schrøder HD, Hejbøl EK, Füchtbauer E-M, de Oliveira JRM, Pedersen L. Loss of function of Slc20a2 associated with familial idiopathic Basal Ganglia calcification in humans causes brain calcifications in mice. J Mol Neurosci MN. 2013;51(3):994–999. doi: 10.1007/s12031-013-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289(5477):265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 61.Szeri F, Lundkvist S, Donnelly S, Engelke UFH, Rhee K, Williams CJ, et al. The membrane protein ANKH is crucial for bone mechanical performance by mediating cellular export of citrate and ATP. PLoS Genet. 2020;16(7):e1008884. doi: 10.1371/journal.pgen.1008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nürnberg P, Thiele H, Chandler D, Höhne W, Cunningham ML, Ritter H, et al. Heterozygous mutations in ANKH, the human ortholog of the mouse progressive ankylosis gene, result in craniometaphyseal dysplasia. Nat Genet. 2001;28(1):37–41. doi: 10.1038/ng0501-37. [DOI] [PubMed] [Google Scholar]
- 63.Reichenberger E, Tiziani V, Watanabe S, Park L, Ueki Y, Santanna C, et al. Autosomal dominant craniometaphyseal dysplasia is caused by mutations in the transmembrane protein ANK. Am J Hum Genet. 2001;68(6):1321–1326. doi: 10.1086/320612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gurley KA, Reimer RJ, Kingsley DM. Biochemical and genetic analysis of ANK in arthritis and bone disease. Am J Hum Genet. 2006;79(6):1017–1029. doi: 10.1086/509881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prosdocimo DA, Douglas DC, Romani AM, O’Neill WC, Dubyak GR. Autocrine ATP release coupled to extracellular pyrophosphate accumulation in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2009;296(4):C828–839. doi: 10.1152/ajpcell.00619.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Costello JC, Rosenthal AK, Kurup IV, Masuda I, Medhora M, Ryan LM. Parallel regulation of extracellular ATP and inorganic pyrophosphate: roles of growth factors, transduction modulators, and ANK. Connect Tissue Res. 2011;52(2):139–146. doi: 10.3109/03008207.2010.491928. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Brown MA, Peach C, Russell G, Wordsworth BP. Investigation of the role of ENPP1 and TNAP genes in chondrocalcinosis. Rheumatol Oxf Engl. 2007;46(4):586–589. doi: 10.1093/rheumatology/kel338. [DOI] [PubMed] [Google Scholar]
- 68.Addison WN, Azari F, Sørensen ES, Kaartinen MT, McKee MD. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem. 2007;282(21):15872–15883. doi: 10.1074/jbc.M701116200. [DOI] [PubMed] [Google Scholar]
- 69.Wang J, Tsui HW, Beier F, Pritzker KPH, Inman RD, Tsui FWL. The ANKH ΔE490 mutation in calcium pyrophosphate dihydrate crystal deposition disease (CPPDD) affects tissue non-specific alkaline phosphatase (TNAP) activities. Open Rheumatol J. 2008;2:23–30. doi: 10.2174/1874312900802010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Tsui HW, Beier F, Tsui FWL. The CPPDD-associated ANKH M48T mutation interrupts the interaction of ANKH with the sodium/phosphate cotransporter PiT-1. J Rheumatol j. 2009;36(6):1265–1272. doi: 10.3899/jrheum.081118. [DOI] [PubMed] [Google Scholar]
- 71.Couto AR, Zhang Y, Timms A, Bruges-Armas J, Sequeiros J, Brown MA. Investigating ANKH and ENPP1 in Slovakian families with chondrocalcinosis. Rheumatol Int s. 2012;32(9):2745–2751. doi: 10.1007/s00296-011-2022-8. [DOI] [PubMed] [Google Scholar]
- 72.Pendleton A, Johnson MD, Hughes A, Gurley KA, Ho AM, Doherty M, et al. Mutations in ANKH cause chondrocalcinosis. Am J Hum Genet. 2002;71(4):933–940. doi: 10.1086/343054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Villa-Bellosta R, Egido J. Phosphate, pyrophosphate, and vascular calcification: a question of balance. Eur Heart J. 2017;38(23):1801–1804. doi: 10.1093/eurheartj/ehv605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not available to be shared.