Abstract
Aim
Hyperfibrinogenemia had been widely observed in various cancer patients, however, whether fibrinogen (FIB) influences the survival outcome of patients with primary liver cancer (PLC) remains unknown. This study was aimed to evaluate the predictive value of preoperative FIB in the survival outcome of PLC patients and explore the possible mechanism.
Methods
Retrospective study was performed in PLC patients who underwent hepatectomy. Logistic regression analysis was used to explore the independent risk factors of the overall survival (OS) of PLC patients. The predictive value of FIB for the survival outcome was analyzed by Kaplan-Meier method, receiver operating characteristic curve and Cox proportional hazard model combined with B-splines. Hepatoma cell migration and invasion were detected by wound healing assay and Transwell assay, protein expression was measured by Western blot. mTOR inhibitor and PTEN overexpression plasmid were used to confirm the involvement of the PTEN/AKT/mTOR pathway during FIB treatment.
Results
Preoperative FIB was confirmed to be related with the OS in PLC patients, higher FIB (>2.5 g/L) indicated higher hazard ratio. Meanwhile, FIB could promote hepatoma cell migration and invasion through the activation of AKT/mTOR pathway and epithelial-mesenchymal transformation (EMT). Moreover, the promotion of FIB on cell migration and invasion could be inhibited by mTOR inhibitor and PTEN overexpression.
Conclusions
Preoperative FIB could be related with the prognosis of PLC patients, the risk of death in PLC patients gradually increases along with the up-regulation of FIB. FIB may promote hepatoma metastasis by inducing EMT via the activation of PTEN/AKT/mTOR pathway.
Keywords: Fibrinogen, Primary liver cancer, Survival, Epithelial-mesenchymal transition, PTEN/AKT/mTOR pathway
1. Introduction
Primary liver cancer (PLC) is one of the malignant tumors with high morbidity and mortality worldwide, and the 5-year survival rate has not significantly improved in recent years. Most patients are already in advanced stage due to lack of specificity in the clinical symptoms of early PLC, resulting in the poor prognosis after hepatectomy. Therefore, it is very important to find suitable indicators that can predict the survival of PLC patients who had undergone surgery.
Previous studies have shown that the abnormal activation of the coagulation and fibrinolytic systems often occur in cancer patients [[1], [2], [3]] which could affect the migration and invasion of tumor cells [4,5]. Hyperfibrinogenemia is very common in patients with malignant tumors and closely related with tumor cell migration, invasion and vascular formation [[6], [7], [8], [9]]. Fibrinogen (FIB), as the coagulation factor with the highest content in plasma, is involved in coagulation and fibrinolysis. FIB plays an important role in cell-matrix interaction, inflammatory response and wound healing [10]. Studies have confirmed that FIB deficiency could affect the tumor metastasis in mice model [11], which might be related with the fact that FIB takes part in maintaining vascular integrity, cell adhesion, tissue repair and trans-endothelial cell migration [12,13]. Kolodziejczyk J et al. hold the opinion that FIB could stimulate endothelium to secrete von Willebrand factor, resulting in activation of platelets accompanying neoplastic disorders. Besides, Fragment E (the end product of FIB degradation) could stimulate proliferation, migration and differentiation of endothelial cells, leading to tumor vasculature [4]. Meanwhile, FIB has been proven to regulate the migration and invasion of tumor cells through epithelial-mesenchymal transition (EMT) by regulating the expressions of Slug and ZEB1 [14].
Therefore, this study aimed to find predictors of the survival outcome of PLC patients who had underwent hepatectomy, and evaluate whether FIB could be used as a potential predictor of the prognosis of PLC patients. As well as exploring the possible molecular mechanism of FIB affecting PLC metastasis.
2. Patients and methods
2.1. Patients
1029 PLC patients who had undergone surgical resection in Huashan Hospital of Fudan University (Shanghai, China) from December 2014 to December 2017 were recruited in this study. All patients were diagnosed by pathological biopsy, CT or MRI and in line with the Asian Pacific Association for the Study of the Liver (APASL) guideline. The following PLC patients were excluded: (1) died during hospitalization; (2) younger than 18 years; (3) clinical data or laboratory test results were incomplete; (4) had other tumors in addition to PLC.
Blood samples were obtained before surgery and plasma FIB concentration was measured in the central laboratory of Huashan hospital by using Sysmex CS5100 fully automatic biochemical analyser (Sysmex, Japan). The normal range was defined at the levels between 2.0 and 4.0 g/L. Based on this, patients were divided into three groups: low FIB group (<2.0 g/L), normal FIB group (2.0–4.0 g/L) and high FIB group (>4.0 g/L).
This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Huashan Hospital of Fudan University (NO. 2019-260). Due to the retrospective nature of the study, informed consent was waived and the patients’ personal information was anonymized.
2.2. Data collection
Baseline information of all patients included was collected through Lianzhong computer system of digitized medical records (Shanghai Lianzhong Network Information Co. LTD, Shanghai, China). All PLC patients were followed up every 3 months for the first 2 year after surgery, every 6 months for the 3rd year after surgery, and annually thereafter until cancer recurrence or death. The clinical data, operation-related indicators, laboratory results and clinical outcome of the patients were collected during follow-up by outpatient review and telephone inquiry. Follow-up was ended in September 2018. The clinicopathological features of PLC patients were shown in Table 1.
Table 1.
The clinicopathological features of PLC patients.
| Total | Low FIB | Normal FIB | High FIB | p | |
|---|---|---|---|---|---|
| FIB (g/L) | 2.80 ± 1.03 | 1.70 ± 0.22 | 2.71 ± 0.53 | 5.14 ± 0.99 | <0.001 |
| Age | 1029 | 163 | 762 | 104 | 0.084 |
| ≤50 | 338(32.8) | 62(38.0) | 244(32.0) | 32(30.8) | |
| >50 | 691(67.0) | 101(62.0) | 518(68.0) | 72(69.2) | |
| Sex | 0.001 | ||||
| Female | 254(24.6) | 22(13.5) | 200(26.2) | 32(30.8) | |
| Male | 775(75.1) | 141(86.5) | 562(73.8) | 72(69.2) | |
| BMI | 23.27 ± 3.10 | 23.13 ± 2.87 | 23.36 ± 3.09 | 22.86 ± 3.47 | 0.261 |
| ALT (U/L) | 41.67 ± 39.03 | 39.94 ± 26.63 | 40.38 ± 38.65 | 53.86 ± 53.74 | 0.003 |
| AST (U/L) | 38.36 ± 32.17 | 34.66 ± 24.48 | 37.01 ± 28.76 | 53.99 ± 54.69 | <0.001 |
| AFP | 0.989 | ||||
| ≤400 | 817(79.2) | 129(79.1) | 605(79.6) | 83(79.8) | |
| >400 | 210(20.3) | 34(20.9) | 155(20.4) | 21(20.2) | |
| ASA score | 0.046 | ||||
| ≤2 | 990(95.9) | 155(95.1) | 739(97.0) | 96(92.3) | |
| >2 | 39(3.8) | 8(4.9) | 23(3.0) | 8(7.7) | |
| Child-Pugh grade | 0.002 | ||||
| A | 1019(99.0) | 160(98.2) | 759(99.6) | 100(96.2) | |
| B | 10(1.0) | 3(1.8) | 3(0.4) | 4(3.8) | |
| ECOG performance status | 0.002 | ||||
| 0 | 951(92.2) | 12(7.4) | 49(6.4) | 17(16.3) | |
| 1–2 | 78(7.6) | 151(92.6) | 713(93.6) | 87(83.7) | |
| Tumor differentiation | 0.255 | ||||
| Ⅰ | 18(1.7) | 3(1.8) | 13(1.7) | 2(1.9) | |
| Ⅱ | 689(66.8) | 114(69.9) | 515(67.6) | 60(57.7) | |
| Ⅲ | 312(30.2) | 43(26.4) | 229(30.1) | 40(38.5) | |
| Ⅳ | 10(1.0) | 3(1.8) | 5(0.7) | 2(1.9) | |
| TNM stage | 1029 | 163 | 762 | 104 | <0.001 |
| Ⅰ | 370(35.9) | 93(57.1) | 263(34.5) | 14(13.5) | |
| Ⅱ | 384(37.2) | 37(22.7) | 293(38.5) | 54(51.9) | |
| Ⅲ | 241(23.4) | 32(19.6) | 185(24.3) | 24(23.1) | |
| Ⅳ | 34(3.3) | 1(0.6) | 21(2.8) | 12(11.5) | |
| HBsAg (+) | 699(67.7) | 131(80.4) | 511(67.1) | 57(54.8) | <0.001 |
| Anti-HCV (+) | 18(1.7) | 3(1.8) | 14(1.8) | 1(1.0) | 0.811 |
| Tumor size | <0.001 | ||||
| ≤5 cm | 687(66.6) | 140(85.9) | 515(67.6) | 32(30.8) | |
| >5 cm | 342(33.1) | 23(14.1) | 247(32.4) | 72(69.2) | |
| Tumor number | 0.209 | ||||
| Solitary | 834(80.8) | 137(84.0) | 608(79.8) | 89(85.6) | |
| Multiple | 195(18.9) | 26(16.0) | 154(20.2) | 15(14.4) | |
| Tumor resection range | <0.001 | ||||
| ≤3 segments | 762(73.8) | 143(87.7) | 566(74.3) | 53(51.0) | |
| >3 segments | 267(25.9) | 20(12.3) | 196(25.7) | 51(49.0) | |
| Cirrhosis (+) | 659(63.9) | 131(80.4) | 476(62.5) | 52(50.0) | <0.001 |
| Portal hypertension (+) | 101(9.8) | 15(9.2) | 66(8.7) | 20(19.2) | 0.003 |
| Vascular invasion (+) | 120(11.6) | 14(8.6) | 85(11.2) | 21(20.2) | 0.011 |
| Satellite focus (+) | 179(17.3) | 20(12.3) | 129(16.9) | 30(28.8) | 0.002 |
| Hepatic portal occlusion (+) | 423(41.0) | 58(35.6) | 315(41.3) | 50(48.1) | 0.125 |
| Intraoperative blood loss (mL) | 390.16 ± 421.39 | 406.38 ± 438.73 | 363.99 ± 387.79 | 556.44 ± 570.19 | <0.001 |
| Preoperative PT (s) | 11.85 ± 3.63 | 12.47 ± 2.36 | 11.55 ± 1.00 | 13.11 ± 10.63 | <0.001 |
| Preoperative APTT (s) | 28.57 ± 10.54 | 30.73 ± 6.59 | 27.53 ± 3.93 | 32.88 ± 29.87 | <0.001 |
| Preoperative Hb (g/L) | 137.42 ± 20.29 | 136.74 ± 23.39 | 138.97 ± 19.17 | 127.16 ± 20.23 | <0.001 |
| Preoperative PLT ( × 109/L) | 169.14 ± 93.10 | 117.55 ± 55.52 | 167.76 ± 84.39 | 260.04 ± 128.25 | <0.001 |
| Preoperative ALB(g/L) | 41.47 ± 4.73 | 41.27 ± 5.16 | 41.94 ± 4.34 | 38.39 ± 5.62 | <0.001 |
| Preoperative GLB(g/L) | 30.01 ± 5.55 | 28.15 ± 5.63 | 29.72 ± 5.00 | 35.01 ± 6.46 | <0.001 |
| Hospital-stay days | 14.67 ± 6.50 | 14.43 ± 6.81 | 14.59 ± 6.47 | 15.69 ± 6.20 | 0.232 |
| OS(%) | 571(55.3) | 96(82.1) | 435(75.3) | 40(54.1) | <0.001 |
2.3. Cells and reagents
Human hepatoma cell lines BEL-7404, SMMC-7721 and HepG2 were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). BEL-7404 and SMMC-7721 cells were cultured in RPMI 1640 medium containing 10% FBS (fetal bovine serum) (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), penicillin (100U/mL) and streptomycin (100 μg/mL; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). HepG2 cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) medium containing 10%FBS and penicillin/streptomycin. Cells were incubated in a humidified incubator at 37 °C under 5% CO2. FIB was purchased from Sigma-Aldrich (St. Louis, MO, USA). All antibodies including PTEN (#9188), p-mTOR (#5536), mTOR (#2983), p-Akt (#4060), Akt (#4685), E-cadherin (#3195), Vimentin (#5741), N-cadherin (#13116) and GAPDH (#2118) were obtained from Cell Signaling Technology (Beverly, MA, USA).
2.4. CCK8 assay
5 × 103 cells were seeded in each well of the 96-well plate and incubated for 24 h. A series concentration of FIB was added to the wells for different time points treatment. 10 μl CCK8 (Sigma-Aldrich) was added and incubated for 0.5 h at 37 °C. Absorbance at 450 nm was measured by the multi-mode detection platform (Molecular Devices, GmbH, Wals, Austria).
2.5. Wound healing assay
Cells (5 × 105) were seeded in each well of the 6-well plate and cultured until a confluent monolayer was formed. A wound was scratched with the 200 μl pipette tip. The medium was replaced and the plate was washed twice with PBS to remove floating cells and then the cells were cultured with complete medium supplemented with FIB. Cell migration was detected by the closure of scratch and the images were obtained by the inverted microscope (DMI 3000B; Leica microsystems GmbH, Wetzlar, Germany).
2.6. Transwell assay
The migration and invasion ability were detected by the polycarbonate membrane transwell inserts (Corning, NY, USA). 5 × 105 Cells in DMEM with 5% FBS were seeded into the upper chamber and the cell invasion ability was measured by the chamber coated with BD Matrigel after treated with FIB. The lower chamber was added with DMEM containing 20% FBS. The cells on the upper insert were removed after 48 h incubation while the cells migrated to the lower side of the insert were fixed and stained with crystal violet. The number of migrating or invading cells were counted in five randomly chosen fields in each well, and imaging and cell counting were observed by a microscope (Olympus, Tokyo, Japan) at 10x magnification. The experiments were performed in triplicate.
2.7. Western blot
Cell lysates were collected by extracting proteins with RIPA lysis buffer containing proteinase inhibitors compound (P2714, Merck KGaA, Darmstadt, Germany). About 40 μg protein was added into each lane of SDS-PAGE gel and transferred electrophoretically onto PVDF membrane. After blocking with 5% non-fat milk, the membranes were incubated with the following primary antibodies overnight at 4 °C: GAPDH (60004-1-Ig, Proteintech Group, Rosemont, USA; 1:10000 dilution), PTEN (22034-1-AP, Proteintech Group, Rosemont, USA; 1:4000 dilution), Cadherin (60004-1-Ig, Proteintech Group, Rosemont, USA; 1:1000 dilution), mTOR (#2983, Cell Signaling Technology, Boston, MA, USA; 1:1000 dilution), p-mTOR (#5536, Cell Signaling Technology, Boston, MA, USA; 1:1000 dilution), AKT (#4685, Cell Signaling Technology, Boston, MA, USA; 1:1000 dilution), p-AKT (#4060, Cell Signaling Technology, Boston, MA, USA; 1:1000 dilution), E-cadherin (#14472, Cell Signaling Technology, Boston, MA, USA; 1:1000 dilution), vimentin (#46173, Cell Signaling Technology, Boston, MA, USA; 1:1000 dilution). The secondary antibody conjugated to HRP (A0208, Beyotime biotechnology, Shanghai, China) was used to visualize the stained bands using ECL kit (Millipore, USA). Band intensity was semi-quantified by Fusion Fx7 (Vilber Lourmat, France).
2.8. PTEN overexpression
Transfection mix was prepared according to the protocol (Thermo Fisher) and added to HEK293T for 48 h incubation. The supernatant of the HEK293T was collected to incubate hepatoma cells. Then puromycin at the killing concentration (1ug/ml) was added to hepatoma cells until all cells in the control plate are dead. Finally, the rest of the cells were collected to confirm the stable expression of PTEN via Western blot.
2.9. Statistical analysis
SPSS Statistics 25.0 (IBM SPSS), Prism 8 (GraphPad Software) and R 3.4.4 statistical Software were used to analyze. Measurement data was expressed as mean ± standard deviation (SD) and the comparison between groups was performed by Student’s t-test. Enumeration data was expressed as "rate or constituent ratio (%)" and Chi-square test was used for comparison. Logistic regression analysis and receiver operating characteristic (ROC) curve were used to analyze the risk factors of the survival outcome of PLC patients. The overall survival rate was compared by Kaplan-Meier method and log-rank test. The Cox proportional hazard model and the B-spline chart were combined to calculate the hazard ratio (HR) and confidence interval (CI). P < 0.05 was considered statistically significant.
3. Results
3.1. The clinicopathological features of PLC patients
The patients were divided into three groups according to the preoperative FIB level: low FIB group (<2.0 g/L), normal FIB group (2.0–4.0 g/L) and high FIB group (>4.0 g/L). We compared the clinical data, operation-related indicators and laboratory results of the three groups, the results showed that there were significant differences in gender, ALT, AST, ASA, Child-Pugh, ECOG, TNM, HBsAg, tumor size, tumor resection range, cirrhosis, portal hypertension, vascular invasion, satellite focus, intraoperative blood loss, PT, APTT, Hb, PLT, ALB and GLB among three groups (P < 0.05). Most importantly, the survival rate of patients in high FIB group was lower than the other two groups (P < 0.001) (Table 1).
3.2. FIB could be an independent factor influencing the prognosis of PLC patients
In order to explore the risk factors that affect the survival outcome of PLC patients, univariate and multivariate logistic regression analysis were conducted (Table 2). The results showed that AFP, ASA, TNM stage, satellite focus, intraoperative blood loss and FIB were independent factors influencing the prognosis of PLC patients (P < 0.05). The higher the FIB concentration, the greater impact it has on the prognosis of PLC patients (OR = 1.256, 95% CI: 1.023–1.542, p = 0.029).
Table 2.
Logistic regression analysis of the influence on prognosis of PLC patients.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age>50 | 1.013 (0.998, 1.027) | 0.089 | 1.014 (0.996, 1.033) | 0.139 |
| Sex (Male) | 1.622 (1.134, 2.320) | 0.008 | 1.380 (0.867, 2.194) | 0.174 |
| BMI | 1.000 (0.999, 1.001) | 0.523 | 1.001 (1.000, 1.002) | 0.072 |
| ALT | 1.009 (1.005, 1.014) | <0.001 | 1.004 (0.997, 1.010) | 0.248 |
| AST | 1.011 (1.006, 1.016) | <0.001 | 1.006 (0.999, 1.013) | 0.107 |
| AFP>400 μg/L | 0.486 (0.330, 0.715) | <0.001 | 1.899 (1.191, 3.027) | 0.007 |
| ASA>2 | 3.273 (1.550, 6.909) | 0.002 | 3.157 (1.267, 7.864) | 0.014 |
| Child-Pugh grade (B) | 3.918 (0.869, 17.695) | 0.076 | 2.148 (0.320, 14.417) | 0.431 |
| ECOG (1–2) | 2.836 (1.646, 4.946) | <0.001 | 1.928 (0.989, 3.757) | 0.054 |
| TNM stage | ||||
| Ⅰ | 0.132 (0.059, 0.296) | <0.001 | 0.228 (0.088, 0.586) | 0.002 |
| Ⅱ | 0.321 (0.148, 0.698) | 0.004 | 0.338 (0.138, 0.831) | 0.018 |
| Ⅲ | 0.707 (0.322, 1.554) | 0.388 | 0.824 (0.334, 2.035) | 0.675 |
| Ⅳ | ||||
| HBsAg (+) | 1.128 (0.802, 1.586) | 0.489 | 1.236 (0.772, 1.980) | 0.377 |
| Anti-HCV (+) | 2.091 (0.656, 6.665) | 0.212 | 1.440 (0.361, 5.737) | 0.605 |
| Tumor number (Multiple) | 1.602 (1.091, 2.351) | 0.016 | 1.312 (0.822, 2.093) | 0.255 |
| Tumor resection range (>3 segments) | 2.787 (1.965, 3.955) | <0.001 | 1.312 (0.803, 1.986) | 0.313 |
| Cirrhosis (+) | 1.210 (0.868, 1.686) | 0.26 | 1.324 (0.849, 2.064) | 0.216 |
| Portal hypertension (+) | 2.816 (1.711, 4.633) | <0.001 | 1.394 (0.752, 2.586) | 0.292 |
| Vascular invasion (+) | 2.493 (1.541, 4.031) | <0.001 | 1.448 (0.809, 2.591) | 0.213 |
| Satellite focus (+) | 2.978 (2.016, 4.399) | <0.001 | 1.677 (1.031, 2.729) | 0.037 |
| Hepatic portal occlusion (+) | 0.870 (0.626, 1.210) | 0.408 | 0.688 (0.465, 1.016) | 0.061 |
| Intraoperative blood loss (mL) | 1.001 (1.001, 1.001) | <0.001 | 1.001 (1.000, 1.001) | 0.005 |
| Preoperative PT (s) | 1.001 (0.963, 1.040) | 0.964 | 0.973 (0.885, 1.069) | 0.566 |
| Preoperative APTT (s) | 1.009 (0.975,1.044) | 0.601 | 0.998 (0.951, 1.046) | 0.918 |
| Preoperative FIB (g/L) | 1.494 (1.285, 1.737) | <0.001 | 1.256 (1.023, 1.542) | 0.029 |
| Preoperative Hb (g/L) | 0.979 (0.971, 0.987) | <0.001 | 0.988 (0.977, 0.999) | 0.036 |
| Preoperative PLT ( × 109/L) | 0.220 (1.001, 0.999) | 0.22 | 0.999 (0.996, 1.001) | 0.283 |
3.3. FIB could be used to predict the survival outcome of PLC patients
ROC curve analysis was used to evaluate the predictive value of preoperative FIB for the survival outcome of PLC patients who had underwent hepatectomy. The results showed that when FIB was used to predict the survival outcome, the area under the curve (AUC) is 0.630 (95%CI: 0.584–0.677, p < 0.001) which was statistically significant, indicating that FIB could be used to predict the survival outcome of PLC patients (Fig. 1).
Fig. 1.
FIB could be used to predict the survival outcome of PLC patients.
3.4. PLC patients with higher FIB had lower survival rates
To explore the correlation between FIB and survival outcome of PLC patients, Kaplan-Meier method and log-rank test were used for analysis. As shown in Fig. 2, there was significant difference in OS among three groups (high FIB group: normal FIB group: low FIB group = 17.20%: 24.48%: 45.21%, P < 0.001). The OS of the high FIB group was significantly lower than the other two groups (P < 0.001), while there was no significant difference between the low FIB group and the normal FIB group.
Fig. 2.
PLC patients with higher FIB had lower survival rates.
3.5. The mortality risk of PLC patients gradually increased with the up-regulation of FIB
To verify the predictive value of FIB for survival outcome of PLC patients and find the optimal cut-off value of FIB, Cox proportional risk model combined with B-spline diagram were used for analysis. As indicated in Fig. 3, the mortality risk ratio of PLC patients was 1.0 when FIB was 2.5 g/L. And when FIB>2.5 g/L, the mortality risk of PLC patients gradually increased with the up-regulation of FIB.
Fig. 3.
The mortality risk of PLC patients gradually increased with the up-regulation of FIB.
3.6. FIB had no significant effect on the proliferation of hepatoma cells
To determine whether FIB participates in the development and progression of PLC, we observed the effect of FIB on the proliferation and viability of hepatoma cells in vitro. Based on previously reported research focused on FIB biological effect [15,16], we have chosen the FIB concentration as 40ug/ml and 80 μg/mL in our study. CCK8 assay showed that FIB did not significantly affect the proliferation and viability of hepatoma cells (Fig. 4A–C).
Fig. 4.
FIB had no significant effect on the proliferation of hepatoma cells. Hepatoma cells were treated with 40 μg/ml and 80 μg/ml FIB for 24 h, 48 h, 72 h and 96 h, respectively (A–C). #, p > 0.05.
3.7. FIB could enhance the migration and invasion ability of hepatoma cells
Wound healing assay showed that the migration of hepatoma cells was enhanced after FIB treatment (40 μg/mL) (Fig. 5A–B). Meanwhile, Transwell assay showed that the numbers of hepatoma cells pass through the chamber (coated with matrigel or not) were increased after FIB exposure (40 μg/mL) (Fig. 5C–D). Therefore, FIB significantly promoted the migration and invasion ability of hepatoma cells.
Fig. 5.
FIB could enhance the migration and invasion abilities of hepatoma cells. Hepatoma cells were treated with FIB (40 μg/mL) for 48 h, wound healing assay and Transwell assay were performed to measure cell migration (A–C) and invasion ability (D). *, p < 0.05. **, p < 0.01. ***, p < 0.001.
3.8. FIB could activate EMT and the PTEN/AKT/mTOR pathway
We detected the expressions of E-cadherin, N-cadherin and Vimentin which are important in EMT, as well as the vital proteins in the PTEN/AKT/mTOR pathway. EMT is a process in which epithelial cells lose their epithelioid characteristics and acquire mesenchymal cell characteristics. Due to the decreased E-cadherin, tumor cells lose cell-to-cell contact and have the ability to spread to adjacent or distant tissues, resulting in the increased expression of N-cadherin [17]. Meanwhile, Vimentin is also a marker of cell migration. The Western blot results showed that the expression of E-cadherin was decreased while N-cadherin and Vimentin were increased significantly upon FIB exposure (40, 80 μg/mL), which indicated FIB might enhance the migration and invasion ability of hepatoma cells by promoting EMT (Fig. 6A–B). As an important signaling pathway, Akt/mTOR pathway can affect cell growth, proliferation, differentiation and apoptosis. Activation of Akt/mTOR pathway not only leads to the malignant transformation of cells, but also participates in the regulation of tumor cell migration and invasion, tumor angiogenesis and extracellular matrix degradation, which are closely related to the occurrence and development of cancer [18]. The increased AKT and mTOR and decreased PTEN after FIB exposure, suggesting FIB could activate the PTEN/Akt/mTOR pathway (Fig. 6C–D).
Fig. 6.
FIB could activate EMT and the PTEN/AKT/mTOR pathway. Western blot was used to detect the expressions of key proteins related with EMT and the PTEN/AKT/mTOR pathway after hepatoma cells were treated with FIB (40 μg/mL and 80 μg/mL) for 24 h. GAPDH was used as a loading. *, p < 0.05. **, p < 0.01. ***, p < 0.001. ns, p > 0.05.
3.9. Blocking mTOR could inhibit the promotion of FIB on the migration and invasion of hepatoma cells
To verify whether FIB promoted the migration and invasion of hepatoma cells by activating the AKT/mTOR pathway, we preincubated hepatoma cells with AZD8055 (mTOR inhibitor) for 24 h, and then treated cells with FIB (40 μg/mL) to observe the alteration of cell migration and invasion, as well as the related proteins. The results showed that the promoting effect of FIB on the migration and invasion of hepatoma cells was inhibited by AZD8055 (Fig. 7A–C). Meanwhile, the expression of E-cadherin was increased while N-cadherin, Vimentin, p-AKT and p-mTOR were all decreased in AZD8055 and FIB combined group compared with FIB alone group (Fig. 7D-E).
Fig. 7.
Blocking mTOR could inhibit the promotion of FIB on the migration and invasion of hepatoma cells. Treating hepatoma cells with FIB (40 μg/mL) for 48 h which were preincubated with AZD8055 (100 μM) for 24 h, then wound healing assay and Transwell assay were performed to measure cell migration and invasion ability (A–C). Western blot was used to detect the expression of related proteins (D–E). GAPDH was used as a loading. Compared to the group on the left, *, p < 0.05. **, p < 0.01. ***, p < 0.001. ns, p > 0.05.
3.10. FIB might activate the AKT/mTOR pathway to promote the migration and invasion of hepatoma cells by inhibiting PTEN
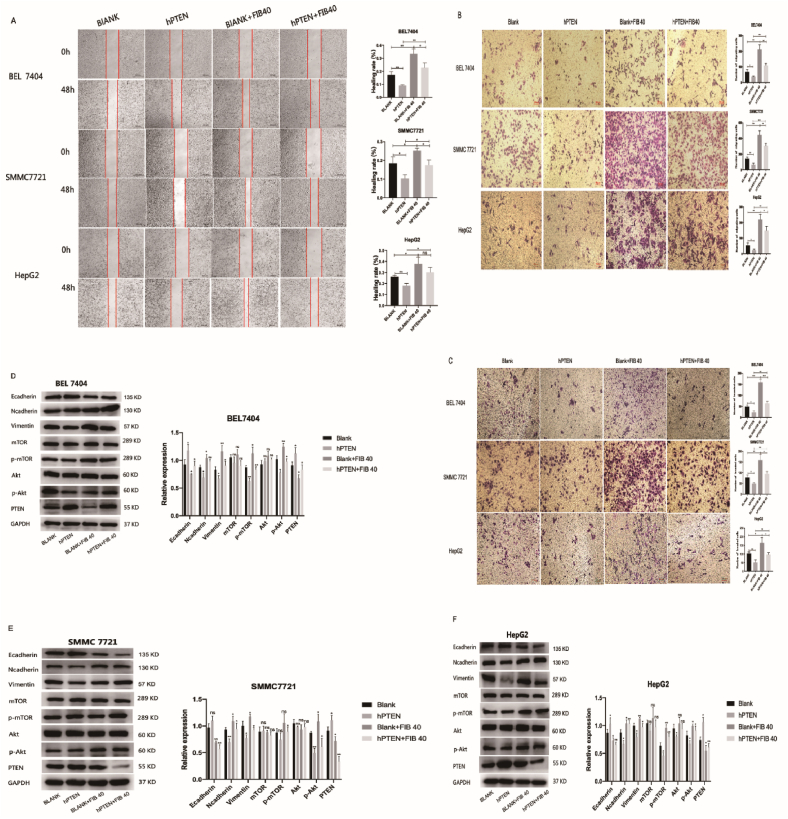
In order to verify whether the activation of AKT/mTOR pathway induced by FIB was related with PTEN, we constructed stable transgenic hepatoma cells overexpressing PTEN and the hepatoma cells infected with empty plasmid were used as the blank group. After PTEN overexpression, the promoting effect of FIB on the migration and invasion of hepatoma cells was significantly inhibited (Fig. 8A–C). Moreover, compared with the FIB-treated blank group, the expression of E-cadherin was increased while N-cadherin, Vimentin, p-AKT and p-mTOR were decreased in the PTEN overexpression group after FIB (40 μg/mL) stimulation (Fig. 8D–F).
Fig. 8.
FIB might activate the AKT/mTOR pathway to promote the migration and invasion of hepatoma cells by inhibiting PTEN. Hepatoma cells were divided into four groups: blank group, PTEN overexpression group, blank group treated with FIB and PTEN overexpression group treated with FIB, respectively. wound healing assay and Transwell assay were performed to measure cell migration and invasion ability (A–C). Western blot was used to detect the expression of related proteins (D–F). GAPDH was used as a loading. *, p < 0.05. **, p < 0.01. ***, p < 0.001. ns, p > 0.05.
4. Discussion
This study confirmed preoperative FIB, AFP, ASA, TNM, satellite focus and intraoperative blood loss were independent factors influencing the OS of PLC patients who had underwent surgery. Among them, FIB had a certain predictive value for the survival outcome, the OS of PLC patients with high FIB was significantly lower. When FIB>2.5 g/L, the risk of death of PLC patients increased gradually with the up-regulation of FIB.
FIB is a 340 KDa soluble glycoprotein synthesized by the liver cell. It consists of three pairs of polypeptide chains, namely fibrinogen alpha chain (FGA), fibrinogen beta chain (FGB) and fibrinogen gamma chain (FGG). Zhu et al. found that FGG was up-regulated in liver cancer cells and tissues, and the increased FIB concentration was closely related with tumor thrombosis [19]. Meanwhile, studies have shown that FIB acts as the acute phase reaction protein in systemic inflammatory response [20], FIB could induce the production of a variety of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) and mediate the interaction between leukocytes and endothelial cells to regulate the inflammatory response [21,22]. The inflammation induced by FIB has been confirmed to be associated with tumor progression [23]. More and more evidence indicated that FIB was involved in different stages of tumor development [24]. FIB could interact with a plenty of cytokines, including transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) to regulate the proliferation, apoptosis and metastasis of tumor cells [25,26].
FIB can bind to platelets to promote its invasion after converting into fibrin by thrombin, in view of some tumor cells express high levels of FIB receptors, including intercellular adhesion molecule-1 (ICAM-1), α5β1 and αvβ3 integrins [18,27]. Collectively, FIB could act as a bridge to promote the adhesion of platelets to the circulating tumor cells (CTCs) [5], then CTCs would escape the clearance of natural killer cells because of the formation of this matrix barrier [28]. Roche et al. also confirmed that FIB mediated the adhesion of endothelial cells to CTCs through ICAM-1 [29]. Therefore, FIB may play an important role in the adhesion between tumor cells, platelets or endothelial cells, and promote hematogenous metastasis of tumor [11,21].
In addition, we hold the opinion that FIB might enhance the migration and invasion of hepatoma cells by triggering EMT. Studies have confirmed that tumor cells could synthesize FIB to induce EMT, therefore promoting tumor migration and invasion [16,30]. Epithelial cells lose their cell polarity and adhesion ability, but gain the ability to migrate and invade through EMT which is the initial step of tumor cell invasion and metastasis [31,32]. The capacity of hepatoma cells to migrate and invade was enhanced after EMT transformation, and from which they acquired the characteristics of cancer stem cells [33].
The AKT/mTOR pathway plays a crucial part in regulating EMT, and its activation is closely related with tumor cell growth, metastasis and vascularization [34,35]. AKT is highly expressed in varieties of tumor tissues. As a serine/threonine kinase, phosphorylated AKT can regulate important downstream molecules, including FOXO, mTOR and GSK3β etc. [36,37]. Especially, mTOR is pivotal in modulating cell growth and protein synthesis [38]. Moreover, PTEN, as one of the most common tumor suppressor genes, inhibits the AKT/mTOR pathway by dephosphorylating PIP3 and regulates the proliferation, migration, invasion and drug resistance of tumor cells. Inhibition of PTEN can activate the downstream AKT/mTOR pathway and promote the EMT of tumor cells [[39], [40], [41]]. Our study demonstrated that under the condition of mTOR inhibition or PTEN overexpression, the promoting effect of FIB on the migration and invasion of hepatoma cells could be significantly inhibited.
5. Conclusions
Our study confirmed that preoperative FIB could be used as an independent influencing factor to predict the survival outcome of PLC patients, and with the up-regulation of FIB, the risk of death in PLC patients gradually increased. In addition, we preliminarily explored the underlying mechanism of higher FIB leading to poor survival outcome in vitro. Namely, FIB enhanced hepatoma cell migration and invasion by inducing EMT via the activation of the PTEN/AKT/mTOR pathway. This study provides theoretical and experimental basis for the treatment and follow-up of PLC patients by targeting FIB. In the future, prospective studies with multi-center, large-sample size and longer follow-up period, as well as animal experiments will be conducted to verify the above conclusions.
Ethics approval
Our research was approved by the Ethics Committee of Huashan Hospital of Fudan University (NO. 2019-260).
Author contribution statement
Qi Zhang; Rong Xia: Conceived and designed the experiments; Wrote the paper.
Xuemeng Qian; Jiajing Cai: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Qi Qi; Jia Han; Xinfang Zhu: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Data availability
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the Natural Science Foundation of China (Grant No. 81570166), the Three-Year Initiative Plan for Strengthening Public Health System Construction in Shanghai (2020-2022) (Grant No. GWV-10.1-XK23) and the Weigao Research Foundation of Chinese Society of Blood Transfusion (Grant No. CSBT-WG-2017-11).
Contributor Information
Rong Xia, Email: xiaronghs@126.com.
Qi Zhang, Email: zhangqihs0451@163.com.
Abbreviations
- FIB
fibrinogen
- PLC
primary liver cancer
- OS
overall survival
- EMT
epithelial-mesenchymal transformation
- APASL
Asian Pacific Association for the Study of the Liver
- SD
standard deviation
- ROC
receiver operating characteristic
- HR
hazard ratio
- CI
confidence interval
- AUC
area under the curve
- FGA
fibrinogen alpha chain
- FGB
fibrinogen beta chain
- FGG
fibrinogen gamma chain
- TGF-β
transforming growth factor-β
- VEGF
vascular endothelial growth factor
- FGF
fibroblast growth factor
- PDGF
platelet-derived growth factor
- ICAM-1
intercellular adhesion molecule-1
- CTCs
circulating tumor cells
References
- 1.Lyman, G.H. and A.A. Khorana, Cancer, Clots and Consensus: New Understanding of an Old Problem. (1527-7755 (Electronic)). [DOI] [PMC free article] [PubMed]
- 2.Qi, Q., et al., Hyperfibrinogen Is Associated with the Systemic Inflammatory Response and Predicts Poor Prognosis in Advanced Pancreatic Cancer. (1536-4828 (Electronic)). [DOI] [PubMed]
- 3.Tas, F., et al., Clinical and Prognostic Significance of Coagulation Assays in Lung Cancer. (1532-3064 (Electronic)). [DOI] [PubMed]
- 4.Kołodziejczyk, J. and M.B. Ponczek, The Role of Fibrinogen, Fibrin and Fibrin(ogen) Degradation Products (FDPs) in Tumor Progression. (1428-2526 (Print)). [DOI] [PMC free article] [PubMed]
- 5.Zheng, S., et al., Platelets and Fibrinogen Facilitate Each Other in Protecting Tumor Cells from Natural Killer Cytotoxicity. (1349-7006 (Electronic)). [DOI] [PMC free article] [PubMed]
- 6.Adams, G.N., et al., Colon Cancer Growth and Dissemination Relies upon Thrombin, Stromal PAR-1, and Fibrinogen. (1538-7445 (Electronic)). [DOI] [PMC free article] [PubMed]
- 7.Shiose, S., et al., Fibrinogen Stimulates in Vitro Angiogenesis by Choroidal Endothelial Cells via Autocrine VEGF. (721-832X (Print)). [DOI] [PubMed]
- 8.Niu, J.Y., et al., Hyperfibrinogenemia Is a Poor Prognostic Factor in Diffuse Large B Cell Lymphoma. (1432-0584 (Electronic)). [DOI] [PubMed]
- 9.Huang, W., et al., Prognostic Significance of Combined Fibrinogen Concentration and Neutrophil-To-Lymphocyte Ratio in Patients with Resectable Non-small Cell Lung Cancer. (2095-3941 (Print)). [DOI] [PMC free article] [PubMed]
- 10.Mosesson, M.W., Fibrinogen and Fibrin Structure and Functions. (1538-7933 (Print)). [DOI] [PubMed]
- 11.Palumbo, J.S., et al., Fibrinogen Is an Important Determinant of the Metastatic Potential of Circulating Tumor Cells. (6-4971 (Print)). [PubMed]
- 12.Fu, S.J., et al., Prognostic Value of Combined Preoperative Fibrinogen and Neutrophil-Lymphocyte Ratio in Patients with Hepatocellular Carcinoma after Liver Transplantation. (1949-2553 (Electronic)). [DOI] [PMC free article] [PubMed]
- 13.Palumbo, J.S., et al., Spontaneous Hematogenous and Lymphatic Metastasis, but Not Primary Tumor Growth or Angiogenesis, Is Diminished in Fibrinogen-Deficient Mice. (8-5472 (Print)). [PubMed]
- 14.Zhang, X., et al., FGG Promotes Migration and Invasion in Hepatocellular Carcinoma Cells through Activating Epithelial to Mesenchymal Transition. (1179-1322 (Print)). [DOI] [PMC free article] [PubMed]
- 15.Zhang, F., et al., Fibrinogen Promotes Malignant Biological Tumor Behavior Involving Epithelial-Mesenchymal Transition via the P-AKT/p-mTOR Pathway in Esophageal Squamous Cell Carcinoma. (1432-1335 (Electronic)). [DOI] [PMC free article] [PubMed]
- 16.Shu Yj Fau - Weng, H., et al., Clinical and Prognostic Significance of Preoperative Plasma Hyperfibrinogenemia in Gallbladder Cancer Patients Following Surgical Resection: a Retrospective and in Vitro Study. (1471-2407 (Electronic)). [DOI] [PMC free article] [PubMed]
- 17.Giannelli, G., et al., Role of Epithelial to Mesenchymal Transition in Hepatocellular Carcinoma. (1600-0641 (Electronic)). [DOI] [PubMed]
- 18.Alzahrani, A.S., PI3K/Akt/mTOR Inhibitors in Cancer: at the Bench and Bedside. (1096-3650 (Electronic)).
- 19.Zhu, W.L., et al., Abnormal Expression of Fibrinogen Gamma (FGG) and Plasma Level of Fibrinogen in Patients with Hepatocellular Carcinoma. (1791-7530 (Electronic)). [PubMed]
- 20.Davalos, D. and K. Akassoglou, Fibrinogen as a Key Regulator of Inflammation in Disease. (1863-2300 (Electronic)). [DOI] [PubMed]
- 21.Languino, L.R., et al., Fibrinogen Mediates Leukocyte Adhesion to Vascular Endothelium through an ICAM-1-dependent Pathway. (92-8674 (Print)). [DOI] [PubMed]
- 22.Jennewein, C., et al., Novel Aspects of Fibrin(ogen) Fragments during Inflammation. (1528-3658 (Electronic)). [DOI] [PMC free article] [PubMed]
- 23.Steinbrecher, K.A., et al., Colitis-associated Cancer Is Dependent on the Interplay between the Hemostatic and Inflammatory Systems and Supported by Integrin alpha(M)beta(2) Engagement of Fibrinogen. (1538-7445 (Electronic)). [DOI] [PMC free article] [PubMed]
- 24.Polterauer, S., et al., Plasma Fibrinogen Levels and Prognosis in Patients with Ovarian Cancer: a Multicenter Study. (1549-490X (Electronic)). [DOI] [PubMed]
- 25.Martino, M.M., et al., Heparin-binding Domain of Fibrin(ogen) Binds Growth Factors and Promotes Tissue Repair when Incorporated within a Synthetic Matrix. (1091-6490 (Electronic)). [DOI] [PMC free article] [PubMed]
- 26.Witsch, E., Y. Sela M Fau - Yarden, and Y. Yarden, Roles for Growth Factors in Cancer Progression. (1548-9221 (Electronic)). [DOI] [PMC free article] [PubMed]
- 27.Desgrosellier, J.S. and D.A. Cheresh, Integrins in Cancer: Biological Implications and Therapeutic Opportunities. (1474-1768 (Electronic)). [DOI] [PMC free article] [PubMed]
- 28.Palumbo, J.S., et al., Platelets and Fibrin(ogen) Increase Metastatic Potential by Impeding Natural Killer Cell-Mediated Elimination of Tumor Cells. (6-4971 (Print)). [DOI] [PubMed]
- 29.Roche, Y., et al., Fibrinogen Mediates Bladder Cancer Cell Migration in an ICAM-1-dependent Pathway. (340-6245 (Print)). [PubMed]
- 30.Sahni, A., et al., Fibrinogen Synthesized by Cancer Cells Augments the Proliferative Effect of Fibroblast Growth Factor-2 (FGF-2). (1538-7933 (Print)). [DOI] [PubMed]
- 31.Lamouille, S., J. Xu, and R. Derynck, Molecular Mechanisms of Epithelial-Mesenchymal Transition. (1471-0080 (Electronic)). [DOI] [PMC free article] [PubMed]
- 32.Lu, W. and Y. Kang, Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. (1878-1551 (Electronic)). [DOI] [PMC free article] [PubMed]
- 33.Caja, L., A. Kahata K Fau - Moustakas, and A. Moustakas, Context-dependent Action of Transforming Growth Factor β Family Members on Normal and Cancer Stem Cells. (1873-4286 (Electronic)). [DOI] [PubMed]
- 34.Marcucci, F., G. Stassi, and R. De Maria, Epithelial-mesenchymal Transition: a New Target in Anticancer Drug Discovery. (1474-1784 (Electronic)). [DOI] [PubMed]
- 35.Larue, L. and A. Bellacosa, Epithelial-mesenchymal Transition in Development and Cancer: Role of Phosphatidylinositol 3' Kinase/AKT Pathways. (950-9232 (Print)). [DOI] [PubMed]
- 36.Soltani, A., et al., Therapeutic Potency of mTOR Signaling Pharmacological Inhibitors in the Treatment of Proinflammatory Diseases, Current Status, and Perspectives. (1097-4652 (Electronic)). [DOI] [PubMed]
- 37.Altomare, D.A. and J.R. Testa, Perturbations of the AKT Signaling Pathway in Human Cancer. (950-9232 (Print)). [DOI] [PubMed]
- 38.Serrano, I., et al., Role of the Integrin-Linked Kinase (ILK)/Rictor Complex in TGFβ-1-Induced Epithelial-Mesenchymal Transition (EMT). (1476-5594 (Electronic)). [DOI] [PubMed]
- 39.Lee, Y.R., M. Chen, and P.P. Pandolfi, The Functions and Regulation of the PTEN Tumour Suppressor: New Modes and Prospects. (1471-0080 (Electronic)). [DOI] [PubMed]
- 40.Luongo, F., et al., PTEN Tumor-Suppressor: the Dam of Stemness in Cancer. LID - 10.3390/cancers11081076 [doi] LID - 1076. (2072-6694 (Print)). [DOI] [PMC free article] [PubMed]
- 41.Kohnoh, T., et al., Hypoxia-induced Modulation of PTEN Activity and EMT Phenotypes in Lung Cancers. (1475-2867 (Print)). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.