Figure 4.
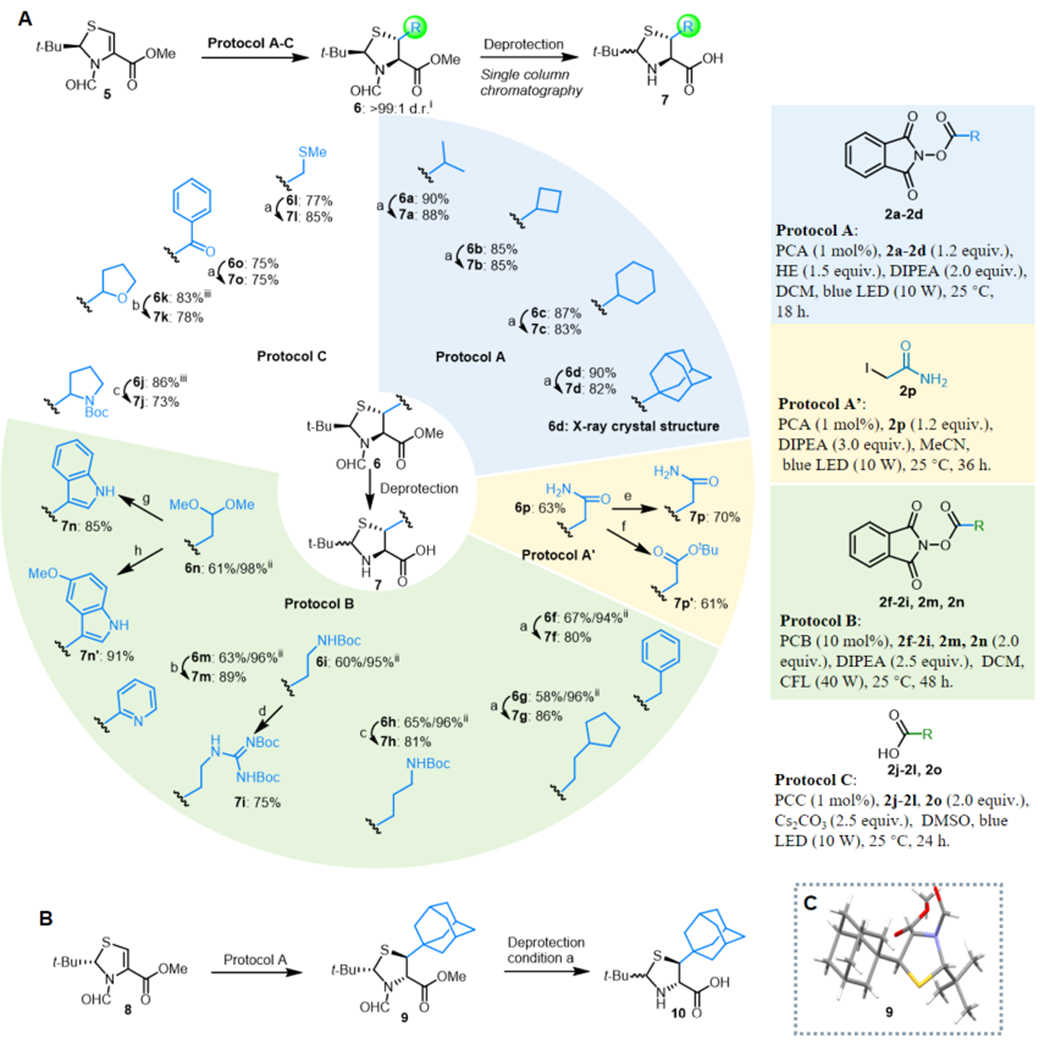
Asymmetric alkylation of thiazoline under photoredox conditions and derivatization. (A) Scope of β-thiolated amino acids. Deprotection conditions: (a) 6 N HCl, 90 °C, 8 h; (b) (1) 0.5 N HCl in MeOH, 25 °C, 12 h, (2) LiOH (10 equiv), H2O/MeOH (1/3 v/v), 25 °C, 6 h; (c) (1) 6 N HCl, 90 °C, 8 h, (2) (Boc)2O (1.0 equiv), DIPEA (2.0 equiv), THF/H2O (10/1 v/v), 25 °C, 1 h; (d) (1) 6 N HCl, 90 °C, 8 h, (2) N,N′-bis-Boc-guanylpyrazole (1.0 equiv), DIPEA (2.0 equiv), MeOH, 25 °C, 3 h; (e) (1) 33% HBr in AcOH, 80 °C, 1 h, (2) LiOH (10.0 equiv), H2O/MeOH (1/3 v/v), 25 °C, 6 h; (f) (1) 6 N HCl, 90 °C, 30 min, (2) p-TsOH (1.0 equiv), isobutylene in DCM (50.0 equiv), 40 °C, 12 h, (3) LiOH (10.0 equiv), H2O/MeOH (1/3 v/v), 25 °C, 6 h; (g) (1) PhNHNH2(2.0 equiv), TFA/DCM (1/3 v/v), 25 °C, 30 min, 97%, (2) 0.5 N HCl in MeOH, rt, 12 h, (3) LiOH (10.0 equiv), H2O/MeOH (1/3 v/v), 25 °C, 6 h; (h) (1) p-MeO-PhNHNH2 (2.0 equiv), TFA/DCM (1/3 v/v), 97%, (2) 0.5 N HCl in MeOH, 25 °C, 12 h, (3) LiOH (10.0 equiv), H2O/MeOH (1/3 v/v), 25 °C, 6 h. (B) Synthesis of d-β-thiolated amino acids from d-cysteine. (C) Absolute configuration of 9 as confirmed by X-ray crystallography. Legend: (i) diastereoselectivity determined by GC analysis of the crude reaction mixture; (ii) brsm, based on recovered starting material; (iii) mixture of diastereomers found.
