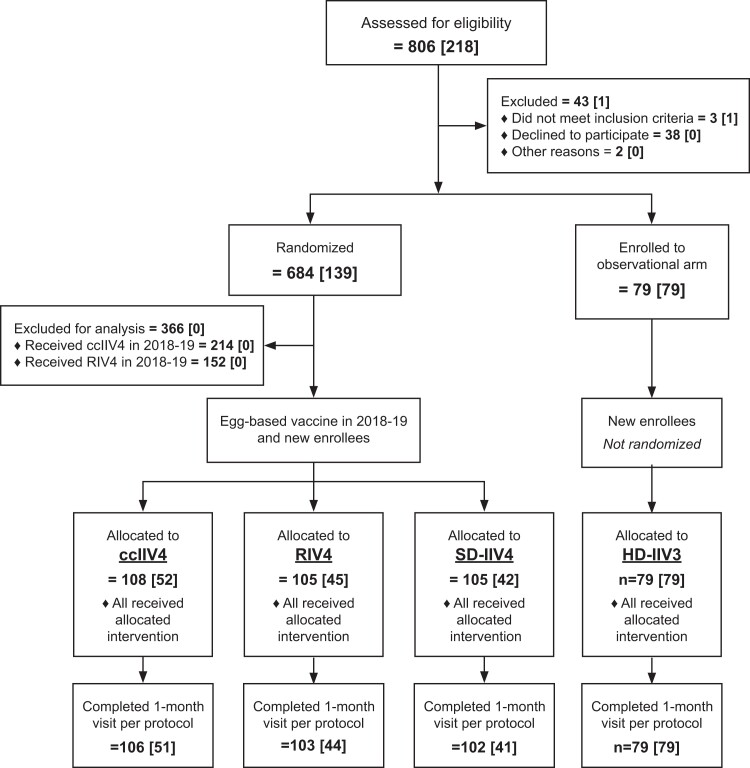
Figure 1.
Screening, enrollment, and follow-up at 1 month of health care personnel aged 18–65 years in an open-label immunogenicity trial of cell culture–based, recombinant, and standard-and high-dose conventional egg-based influenza vaccines, 2019–2020 influenza season. “n” indicates total number of participants per level (number of newly enrolled season 2 participants at each level). Abbreviations: ccIIV4, cell culture–based inactivated influenza vaccine represented by Flucelvax Quadrivalent by Seqirus; HD-IIV3, high-dose trivalent inactivated influenza vaccine represented by Fluzone High-Dose by Sanofi Pasteur; RIV4, recombinant inactivated influenza vaccine represented by Flublok Quadrivalent by Sanofi Pasteur; SD-IIV4, standard-dose quadrivalent inactivated influenza vaccine represented by Fluzone Quadrivalent by Sanofi Pasteur.