Figure 3: hPSC-derived Schwann cells myelinate hPSC-derived sensory neurons and engraft in injured rat sciatic nerves.
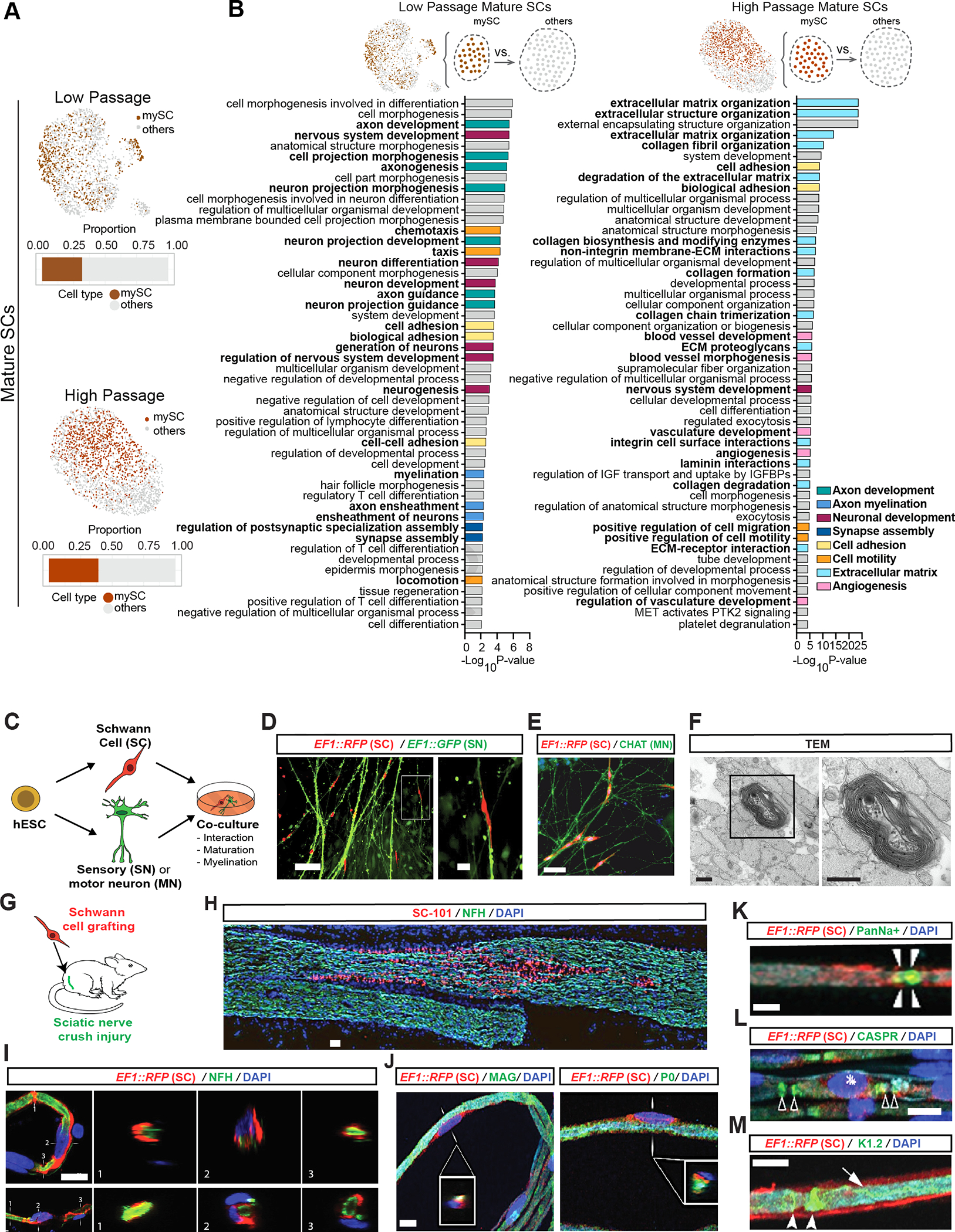
A) Feature plots of mature SC clusters isolated from LP (top) and HP (bottom) scRNA-seq data. dark colors: myelinating (mySC), bar plots: relative population of mySCs.
B) Pathway enrichment analysis of top 250 DEGs of myelinating mature SCs in LP (left) and HP (right). Top 50 pathways from combined gene ontology biological process (GOBP), Reactome and KEGG analysis.
C) Schematic of the SC co-cultures with sensory or motor neurons.
D, E) Immunofluorescence imaging of SCs (EF1::RFP) co-cultured with sensory (EF1::GFP,D) or motor neurons (stained for CHAT,E).
F) TEM of myelin in sensory neuron and SC co-cultures. Images were taken at 80 kV. Scale bars= 500 nm.
G) Schematic of SC transplantation in adult rat sciatic nerves. RFP+ SCs were injected following nerve crush at the site of injury.
H) Immunofluorescence staining of grafted sciatic nerves for human specific nuclear marker SC101 at 8 weeks post transplantation.
I and J) Confocal analysis of teased sciatic nerve fibers for RFP (grafted human cells), axonal marker (NFH, I), myelin markers (MAG and P0, J), node markers Pan-Na+ (sodium channel, arrow heads, K), CASPR (arrow heads, L) and Kv1.2 (K+ channel, arrow heads, M) and DAPI. Scale bars= 100 μm in D left, E and B 20 μm in D right, I and J, 10 μm in K-M.
