Abstract
The field of environmental DNA (eDNA) is advancing rapidly, yet human eDNA applications remain underutilized and underconsidered. Broader adoption of eDNA analysis will produce many well-recognized benefits for pathogen surveillance, biodiversity monitoring, endangered and invasive species detection, and population genetics. Here we show that deep-sequencing-based eDNA approaches capture genomic information from humans (Homo sapiens) just as readily as that from the intended target species. We term this phenomenon human genetic bycatch (HGB). Additionally, high-quality human eDNA could be intentionally recovered from environmental substrates (water, sand and air), holding promise for beneficial medical, forensic and environmental applications. However, this also raises ethical dilemmas, from consent, privacy and surveillance to data ownership, requiring further consideration and potentially novel regulation. We present evidence that human eDNA is readily detectable from ‘wildlife’ environmental samples as human genetic bycatch, demonstrate that identifiable human DNA can be intentionally recovered from human-focused environmental sampling and discuss the translational and ethical implications of such findings.
Subject terms: Ecological genetics; Zoology; Sequencing; Science, technology and society
The recovery of human genomic data from environmental DNA samples raises ethical questions regarding consent, privacy, surveillance and data ownership, which will need to be grappled with as the environmental DNA field moves forward.
Main
The field of environmental DNA (eDNA) research has been rapidly expanding in recent years, resulting in unprecedented advances in a range of biological monitoring applications. Environmental DNA research provides a non-invasive and cost-effective approach for the study and management of wild populations and invasive species, by using a forensics approach to the extraction and identification of DNA fragments released as organisms travel through and interact with the environment1–7. Environmental DNA analysis is also being applied to issues of human and animal health—for example, in pathogen, parasite and pollen monitoring1,8–10. This includes the rapidly emerging field of human eDNA-based pathogen detection from human wastewater. Such approaches developed quickly during the early stages of the COVID-19 pandemic and have already been repurposed for other pathogens such as monkeypox, poliovirus and tuberculosis1,11–15. Environmental DNA has been successfully obtained from a range of sample types including air, soil, terrestrial and aquatic sediments, water (marine, freshwater and wastewater), permafrost, snow and ice cores10,16,17.
Environmental DNA research has traditionally relied primarily on targeted methodologies, such as quantitative PCR (qPCR) and metabarcoding-based next-generation sequencing, and early applications focused on bacterial communities18. However, continued improvements in deep sequencing technology and novel bioinformatics refinements mean that untargeted shotgun-sequencing-based approaches are becoming feasible (Extended Data Fig. 1a), which more fully capture the true extent of genetic diversity within a sample1,8,19. Shotgun sequencing is set to become more labour- and cost-effective than qPCR or metabarcoding in the near future while providing the least biased biodiversity assessments, thus providing the broadest possible presence and abundance information across all taxa. We have recently shown that untargeted shotgun deep sequencing (the direct sequencing of total eDNA with no prior enrichment or selection) can provide both host and pathogen sequence data8,17, while also simultaneously capturing all other biodiversity within an environmental sample. Similar to biodiversity assessments, shotgun sequencing of wastewater samples could be applied to monitor all human pathogens simultaneously but would also probably capture a large volume of human genomic data.
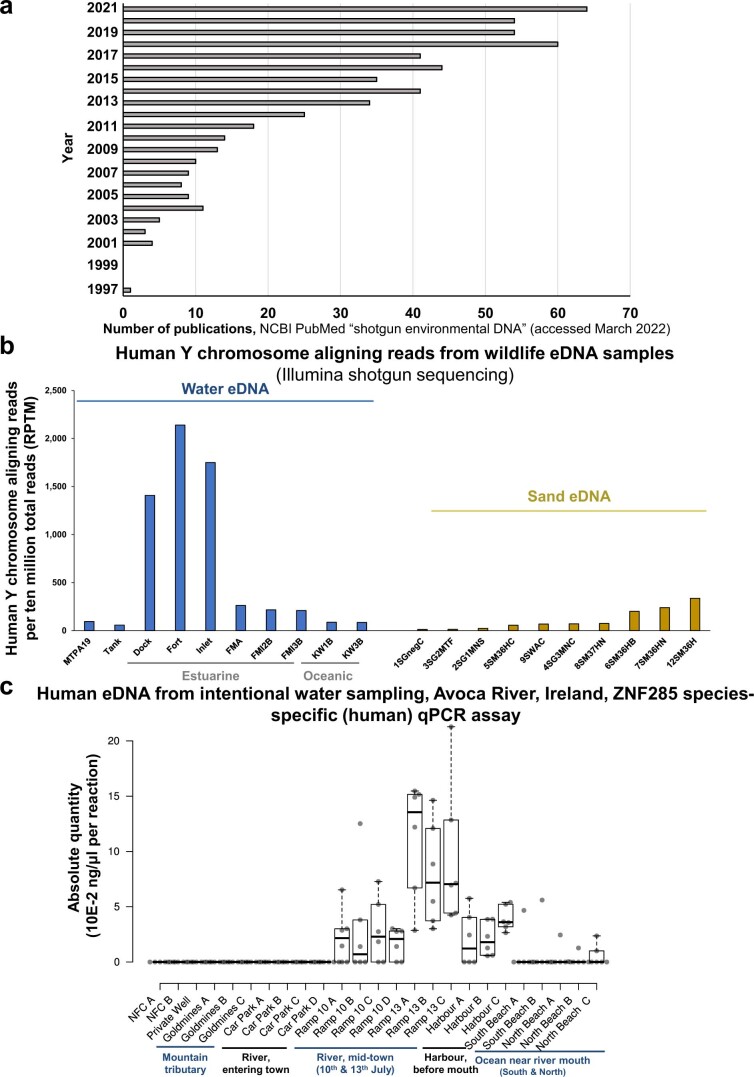
Extended Data Fig. 1. Additional eDNA publication number, human eDNA bycatch and intentional human eDNA quantification data.
1a) Graph of ‘shotgun environmental DNA’ papers in PubMed by year published. A literature search for ‘shotgun environmental DNA’ returned 499 results in NCBI’s PubMed (https://pubmed.ncbi.nlm.nih.gov) and 654 results in Clarivate’s Web of Science (www.webofscience.com). Search conducted on March 11th 2022. Note, papers from 2022 were excluded from the graph, as the year had only partially elapsed, but were included in the total tally. 1b) Human Y chromosome aligning reads per ten million total reads (RPTM) from bycatch shotgun sequenced water and sand eDNA samples. 1c) qPCR based species-specific quantification of human eDNA from Avoca River water sampling, intentional capture. Absolute quantity (10E-2 ng/μl per reaction) of human eDNA per sample. Each qPCR reaction is a 10 μl reaction containing 1 μl of extracted eDNA template. Quantified with ZNF285 human specific assay. For filtered water volumes and elution volumes see Supplemental Table 2. For matching samples quantified with LILRB2 human specific assay see Fig. 1c.
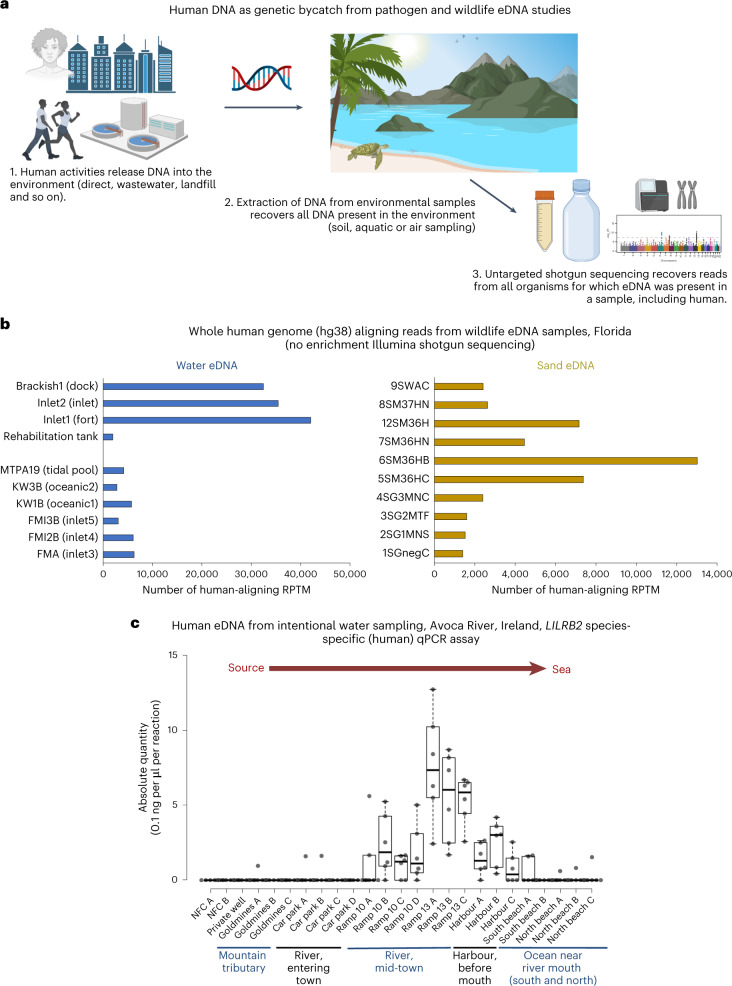
While there is a plethora of beneficial applications of eDNA, we postulate that an unintended negative consequence of eDNA approaches might be the capture of human genomic information (human genetic bycatch (HGB); Fig. 1a). Beneficial applications of human-focused eDNA sampling can also be envisaged. Currently, human DNA is rarely (if ever) the intended target of eDNA studies, leaving the field with a lack of specific human-related regulatory guidelines or ethical approvals. Current targeted qPCR and metabarcoding-based eDNA approaches do not recover any substantial human genomic information. However, as eDNA shifts towards shotgun sequencing, potentially large volumes of human eDNA will be retrieved, including sufficient data to identify and phenotype human individuals. Obtaining genetic data from identifiable persons requires informed consent20. Legal and ethical frameworks are common in studies involving humans and studies that generate patient data, albeit with continued debate regarding whether such policies are sufficiently rigorous in relation to informed consent, data ownership and data protection20–24.
Fig. 1. Recovery of human eDNA from field samples.
a, Schematic overview of how human DNA can enter the environment and be inadvertently sequenced as HGB from pathogen- and wildlife-focused eDNA studies. Schematic created with BioRender. b, Whole human genome aligning reads from a wildlife eDNA shotgun Illumina sequencing study. c, qPCR-based species-specific quantification of human eDNA from Avoca River water sampling. The absolute quantity (0.1 ng per μl per reaction) of human eDNA per sample is shown. Each qPCR reaction is a 10 μl reaction containing 1 μl of extracted eDNA template. The samples were quantified with LILRB2 human-specific assays. For filtered water volumes and elution volumes, see Supplementary Table 2. For matching samples quantified with ZNF285 human-specific assays, see Extended Data Fig. 1c. Tukey whiskers (extend to data points that are less than 1.5 × interquartile range (IQR) away from 1st/3rd quartile) were utilized for each boxplot. The median for each sample is shown as a horizontal line within each box, and box edges are the upper and lower quartiles. One box is graphed per single sample, consisting of all qPCR technical replicate wells for that sample. Biological replicates are not pooled on any boxplots, with each sample being denoted by its own box.
To ascertain whether human genomic DNA could be harvested from eDNA data, we aligned the sequencing data previously generated8,17 as part of our wildlife and pathogen eDNA projects against the human reference genome. Having demonstrated the occurrence of HGB, we next applied species-specific qPCR to quantify the level of human eDNA in environmental water samples from sites distant from and close to human habitation, from human footprints in beach sand and from occupied and unoccupied room air (Supplementary Fig. 1). Finally, we applied long-read shotgun sequencing and short-read sequencing human exome enrichment to obtain human-aligning sequences to reconstruct informative human haplotypes (genetic ancestry and mutations) from eDNA.
Results
Human-aligning reads were detected in all samples (Fig. 1b and Supplementary Table 1) of untargeted shotgun deep sequencing from water and sand eDNA generated for wildlife and pathogen monitoring8,17. Furthermore, in some wild (non-rehabilitation tank) water samples, human-aligning reads were detected at levels almost as high as that of our main study species, the green sea turtle17 (green sea turtle reads per ten million total reads (RPTM)/human RPTM per wild water sample average ratio, 1.39; minimum ratio, 1.03; maximum ratio, 2.54). As expected, the rehabilitation sand and tank water samples had more sea turtle eDNA than human eDNA present (turtle RPTM/human RPTM ratio, 63.50 and 18.50, respectively). The high rate of human eDNA recovered from wild samples (Fig. 1b and Supplementary Table 1) is particularly relevant as our study sites were not directly adjacent to areas of dense human habitation (that is, cities or towns). The relative paucity of human-aligning eDNA reads in the water negative field control and the rehabilitation tank samples (Supplementary Table 1) confirms that the vast majority of human-aligning reads did not originate from contamination during sample processing. In total, 1.8 million paired-end human-aligning reads (300 base pairs (bp) per read pair) were recovered as by-catch from this eDNA study (Fig. 1b and Supplementary Table 1).
The human Y chromosome is a fast-evolving reduced chromosome, which is divergent in structure and gene content from even humans’ closest extant relative, the chimpanzee25. Y chromosomes are not shared among all vertebrate species; they are specific to therian mammals. Sea turtles (our target study animal) do not possess Y chromosomes, instead having temperature-dependent sex determination. The human Y chromosome is therefore a useful genome region for confirming the presence of genuine human reads in these complex metagenomic eDNA sequencing samples. Human Y chromosome genomic bycatch reads were detected in all samples (Extended Data Fig. 1b), despite the Y chromosome’s small size and the default exclusion of reads originating from human females. Estuarine samples tended to have higher human Y chromosome RPTM values than oceanic samples (Extended Data Fig. 1b).
Having determined that HGB can occur from wildlife eDNA sampling, we next investigated the feasibility of intentional human eDNA recovery from sampling sites of suspected high and low human eDNA release. Species-specific qPCR assays for the quantification of human eDNA revealed that human DNA was readily recoverable from water sites located close to towns (Figs. 1c and 2 and Extended Data Figs. 1c, 2 and 3). These findings were replicated in both subtropical Florida and temperate Ireland. Minimal human eDNA was detected in a mountain tributary (Goldmines River) of the Avoca River, Co. Wicklow, Ireland, close to its source and above the line of human habitation (Fig. 1c, Extended Data Fig. 1c and Supplementary Table 2). In contrast, human eDNA was detected once the Avoca River enters Arklow town, with human eDNA levels in the river increasing as it flows through the town, before being diluted when the river enters the Irish Sea (Fig. 1c and Extended Data Figs. 1c and 2a,b). More human eDNA was present in the samples within the town even though less than half of the volume of water could be filtered compared with the non-town samples, due to increased filter clogging induced by more turbid water (Supplementary Table 2). Similarly, no human eDNA was detected from a private well, above the human habitation line (Fig. 1c and Extended Data Fig. 1c). Non-human eukaryotic eDNA was recovered from all Irish samples, demonstrating successful eDNA extraction (Extended Data Fig. 3). Similar to the Irish samples, human eDNA was readily detectible from water samples taken near the city of St. Augustine, Florida, while no human eDNA was detectible by qPCR from ocean water collected on an incoming tide from the ocean beyond the Matanzas Inlet, Florida (Fig. 2a and Extended Data Fig. 4).
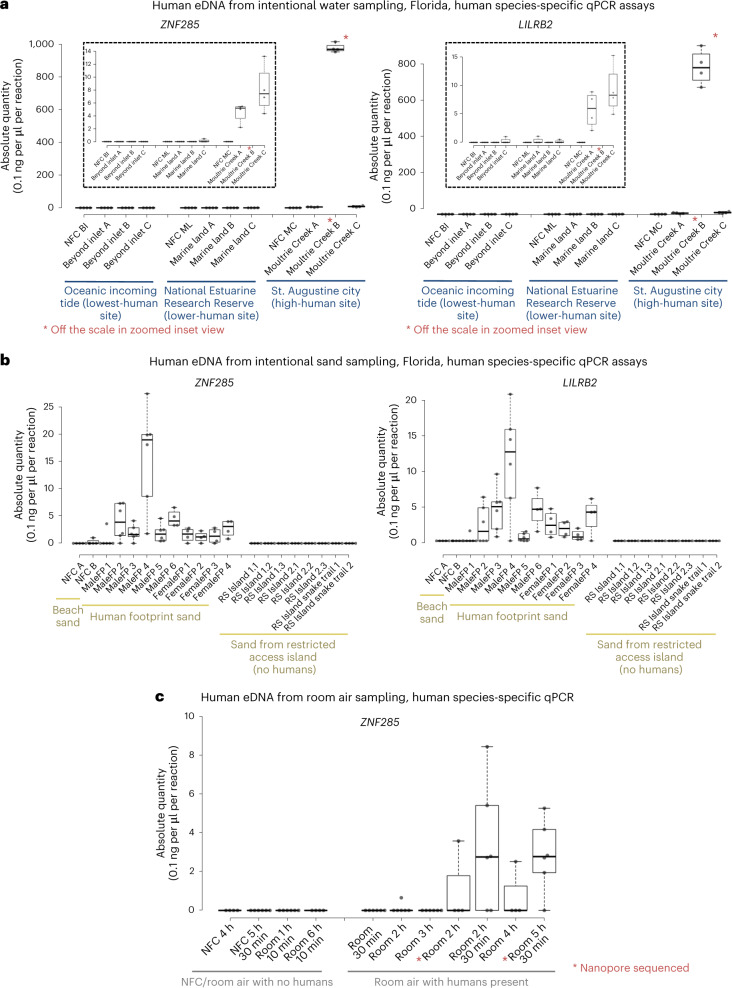
Fig. 2. qPCR-based species-specific quantification of human eDNA from Florida water, sand and air sampling.
a, Water eDNA sampling, qPCR, quantified with ZNF285 (left) and LILRB2 (right) human-specific assays. Insets: enlarged section plot. BI, beyond inlet; ML, marine land; MC, Moultrie Creek. b, Sand eDNA sampling, qPCR, quantified with ZNF285 (left) and LILRB2 (right) human-specific assays. c, Room air eDNA sampling, qPCR, quantified with ZNF285 human-specific assays. The absolute quantity (0.1 ng per μl per reaction) of human eDNA per sample is shown. Each qPCR reaction is a 10 μl reaction containing 1 μl of extracted eDNA template. For filtered water volumes and elution volumes, see Supplementary Table 2. Tukey whiskers (extend to data points that are less than 1.5 × IQR away from 1st/3rd quartile) were utilized for every boxplot. The median for each sample is shown as a horizontal line within each box, and box edges are the upper and lower quartiles. One box is graphed per single sample, consisting of all qPCR technical replicate wells for that sample. Biological replicates are not pooled on any boxplots, with each sample being denoted by its own box.
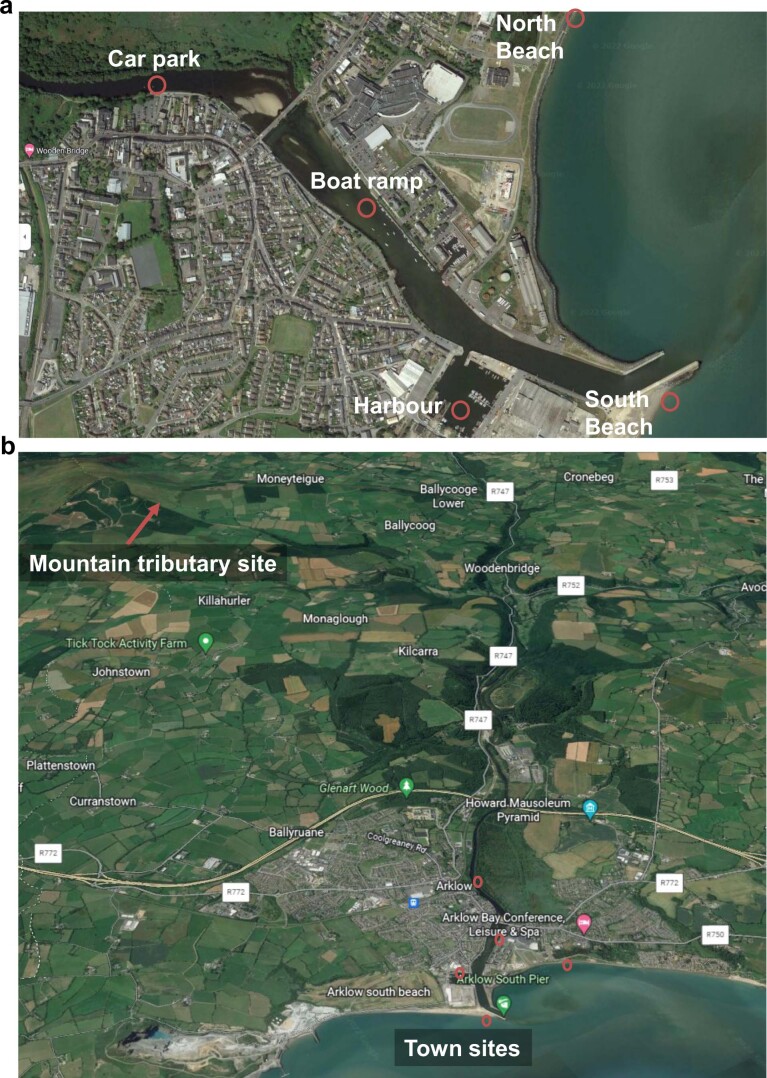
Extended Data Fig. 2. Satellite imagery view (Google Earth), showing the locations of the Irish 2022 sampling sites.
Satellite imagery view (Google Earth, Map data ©2021 Google, SIO, NOAA, U.S Navy, NGA, GEBCO and TerraMetrics), showing the locations of the Irish 2022 sampling sites. 2a) Zoomed view of the Avoca River passing through Arklow Town, Co. Wicklow. 2b) Overview of the full water course from the Goldmines River tributary to the mouth of the Avoca River, Co. Wicklow. The Goldmines River joins the Avoca River, at Woodenbridge, Co. Wicklow.
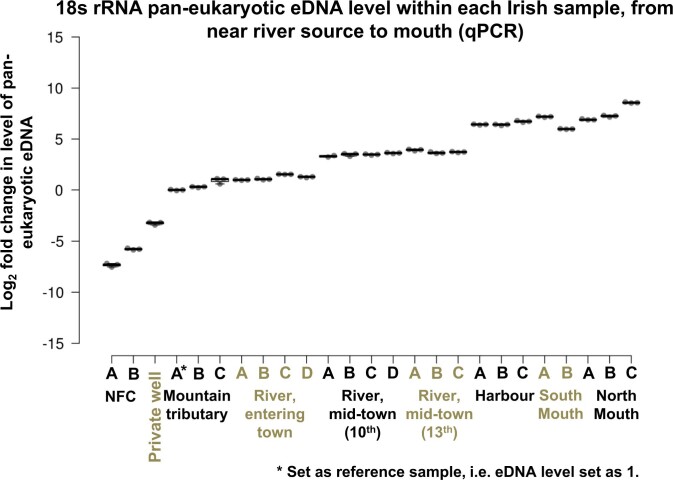
Extended Data Fig. 3. Pan-eukaryotic eDNA levels within each Irish water eDNA sample.
Relative Quantity of 18 s rRNA pan-eukaryotic eDNA levels within each Irish eDNA sample, from near river source to mouth, as assessed by qPCR. Each qPCR reaction is a 10 μl reaction containing 1 μl of extracted eDNA template. For filtered water volumes and elution volumes see Supplemental Table 2.
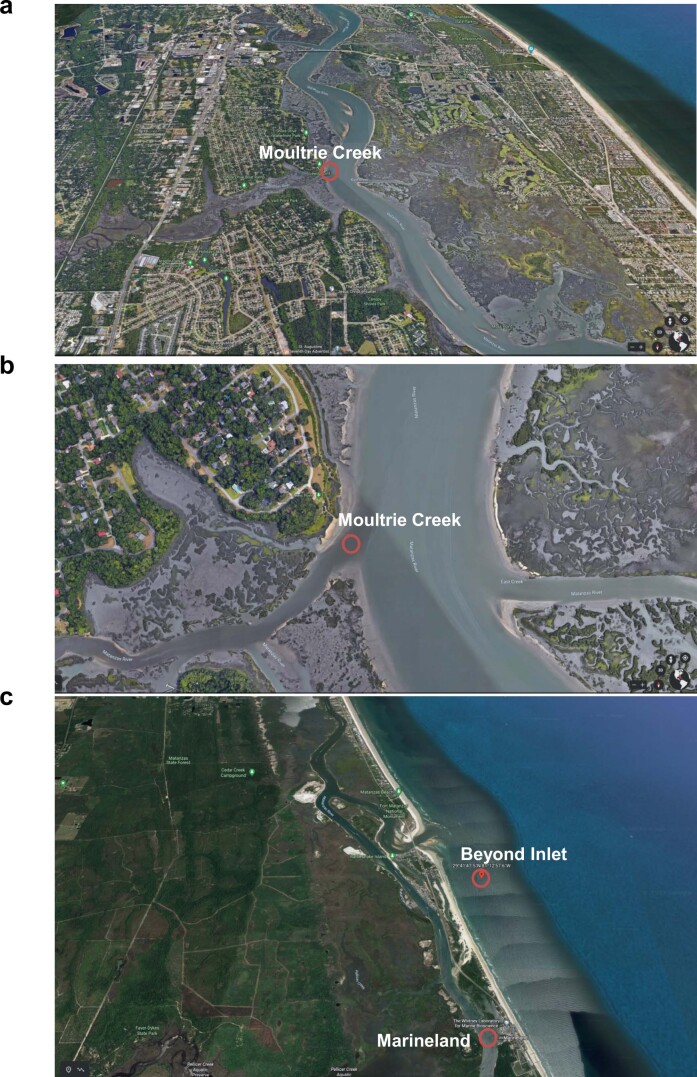
Extended Data Fig. 4. Satellite imagery view (Google Earth), showing the locations of the Florida 2022 sampling sites.
Satellite imagery view (Google Earth, Map data ©2021 Google and TerraMetrics), showing the locations of the Florida 2022 sampling sites. 4a) Overview of the Moultrie Creek sampling site, with surrounding human habitation of the city of St. Augustine visible. 4b) Zoomed view of the Moultrie Creek sampling site. 4c) Overview of the Beyond inlet and Marineland sampling sites.
We next compared human eDNA recovery from beach sand footprints with that from an island with restricted human access. No human eDNA was detected by qPCR in sand samples from a remote area of Rattlesnake Island, Florida, part of the Fort Matanzas National Monument (Fig. 2b and Extended Data Fig. 5). This part of the island is inaccessible to the general public; our access was facilitated by the US National Park Service. By contrast, human eDNA was readily detectible from beach sand samples recovered from human footprints (Fig. 2b).
Extended Data Fig. 5. Restricted human access sand sampling site.
5a) Photo of Rattlesnake (RS) Island site 2, at the Fort Matanzas National Monument, managed by the US National Park Service (NPS). 5b) Satellite imagery (Google Earth, Map data ©2021 Google and TerraMetrics) of the Rattlesnake Island sampling sites. Note that sand samples were also taken from a snake track in the sand at site 2.
Then, we compared the recovery of human eDNA from air in rooms where humans were present with that from air in rooms where humans were absent. No human eDNA was detected by qPCR in negative field controls (eDNA filters left in rooms with humans present during sampling, but not connected to a vacuum pump) or from vacuum-pumped air from rooms in which no humans were present (Fig. 2c). Human eDNA was recovered from rooms with humans present as participants went about regular working activities (Fig. 2c). Human eDNA was recovered even though air samples from rooms with humans present were collected from a sterile veterinary hospital environment.
Differences in the level of human eDNA detected were broadly in close agreement between the two independent species-specific eDNA assays (LILRB2 and ZNF285). This indicates that both are suitable for qPCR-based human eDNA applications (Figs. 1c and 2a,b and Extended Data Fig. 1c).
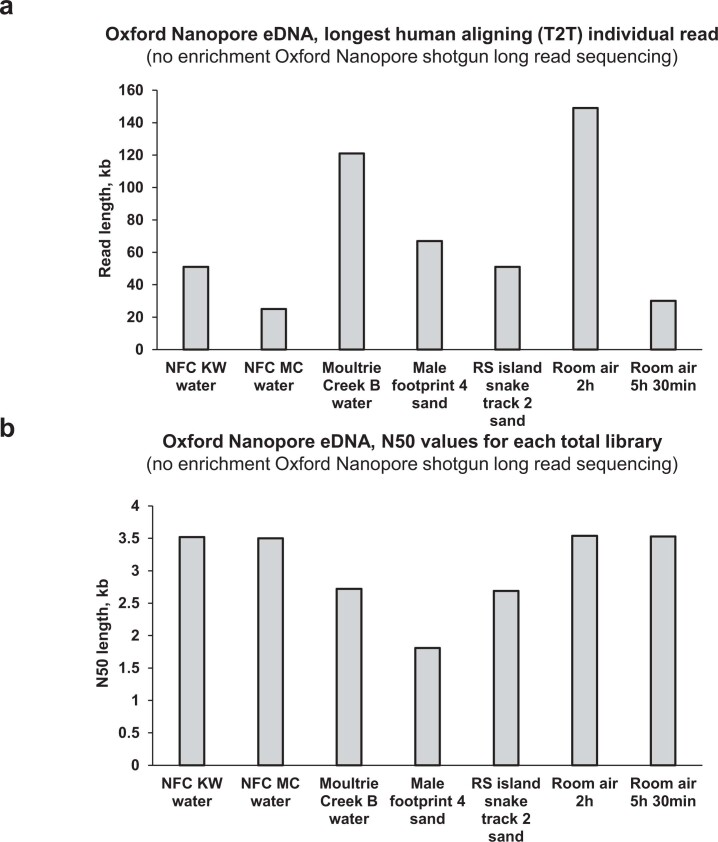
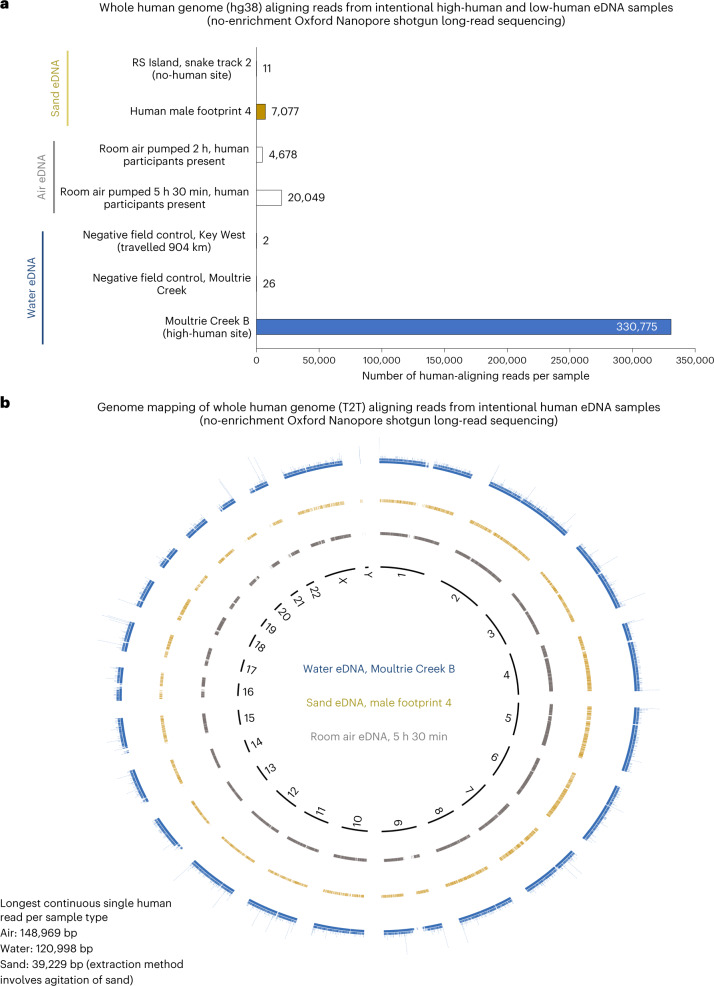
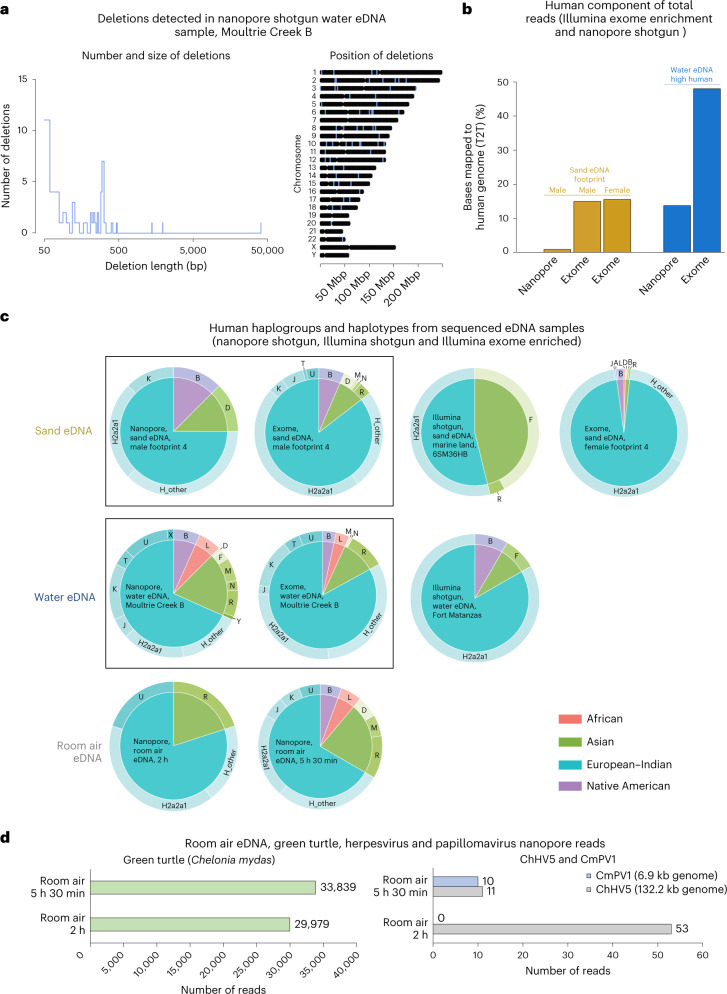
Having quantified the level of human eDNA in each sample by qPCR, we selected seven samples for Oxford Nanopore MinION sequencing (Supplementary Table 3) to confirm that human genomic information could be derived from intentional human eDNA sampling. MinION shotgun sequencing was conducted with six intentional human eDNA samples (collected in 2022) and our longest-travelled water negative field control sample (collected in 2018, travelled over 904 km). Oxford Nanopore sequencing was selected as it generates longer read lengths (Extended Data Fig. 6a,b) than Illumina sequencing. Without any enrichment (shotgun sequencing), the samples of human eDNA in water, human footprint sand and room air returned thousands of human-aligning reads, while negative field control water and no-human-site sand eDNA samples (from a snake trail) had only 2 to 26 human-aligning reads (Fig. 3a and Supplementary Table 3). For all human-positive substrates, coverages were relatively even across the human reference genome, with reads from all chromosomes being detected (Fig. 3b). Nanopore sequencing revealed that eDNA is not necessarily fragmented DNA, as even without employing high-molecular-weight extraction methodology or long-read library preparation protocols (short-read nanopore buffer was used), we recovered human eDNA single reads up to 148,969 bp long (air eDNA; 120,998 bp for water and 39,229 bp for sand) (Fig. 3b and Supplementary Table 4). The longest human-mitochondrial-aligning single read was 16,535 bp, only 34 bp shorter than the full-length mitochondrial reference genome (Supplementary Table 4). The average read length across all human-positive nanopore samples was 1,514 bp (Supplementary Table 4).
Extended Data Fig. 6. Oxford Nanopore long read shotgun sequencing study of eDNA samples post human DNA qPCR-based quantification.
6a) Longest individual human reference genome (T2T) aligning read (in kb) generated from each nanopore sequenced sample. 6b) N50 length (in kb) from each nanopore sequenced sample, for total sequenced reads, that is pre any reference genome alignment. Note, negative field controls had reduced overall numbers of reads (not reflected in N50 values) reflecting a lack of DNA in the sample (Supplemental Table 3).
Fig. 3. Oxford Nanopore long-read shotgun sequencing of eDNA samples.
a, Total number of human-aligning reads (Bowtie2) from each sequenced field, room and negative field control eDNA sample. b, Human genome alignment map of the human-aligning reads (minimap2) from the high-human-site eDNA water sample (Moultrie Creek B), the human male footprint 4 eDNA sample and the room air 5 h 30 min eDNA sample.
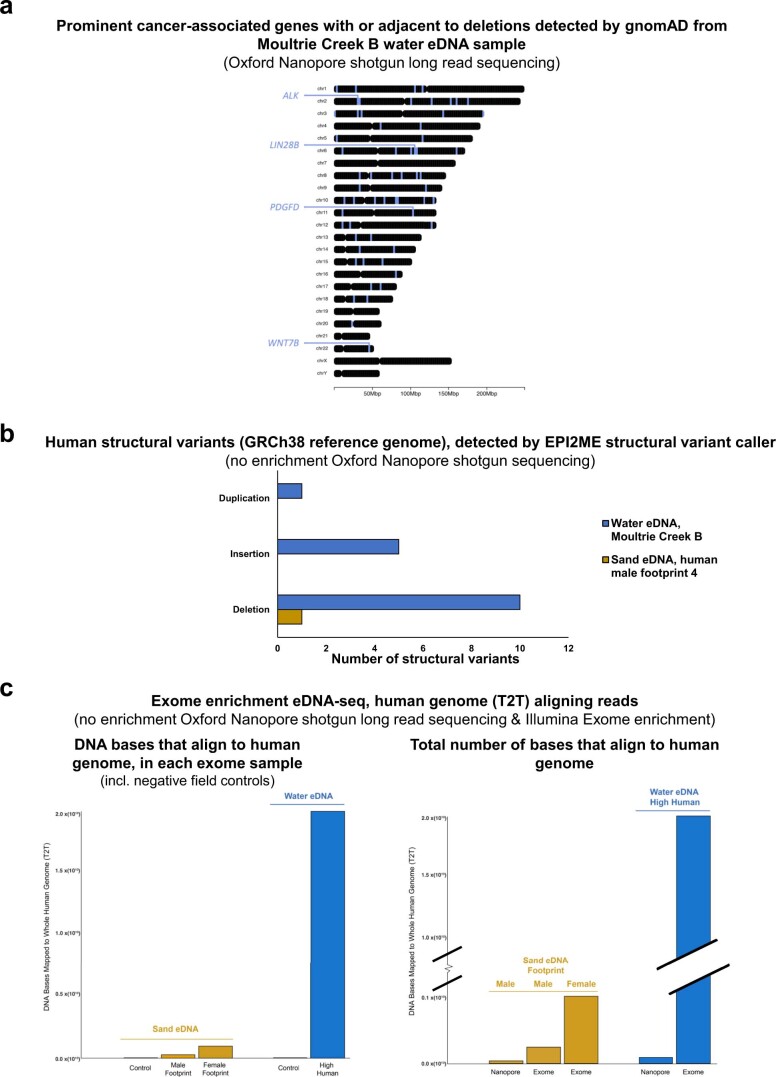
To demonstrate that human eDNA could be used for applications beyond mere quantification, we examined known human genetic variants in the nanopore eDNA data to determine whether eDNA-based ancestry and disease susceptibility applications may be feasible. Deletions annotated in gnomAD (v.2.1)26 could be detected in all three shotgun human-positive eDNA substrates, mainly in water but also to a lesser extent in sand and air samples (Fig. 4a and Supplementary Table 5). The longest deletion detected within a single read was 40,738 bp, a common copy-number polymorphism in European and Latino populations, located on human chromosome 2 (Fig. 4a). Genes associated with the deletions (deletions within or adjacent to the gene) from the Moultrie Creek B water sample have a range of functions including neuron differentiation, the regulation of double-strand break repair and the regulation of proteolysis. Deletions in or near prominent cancer-associated genes (for example, ALK, LIN28B, PDGFD and WNT7B) were also detected (Extended Data Fig. 7a and Supplementary Table 5). Other structural variant types (insertions and duplications) were also detectable, when analysed with the EPI2ME Sniffles-based structural variant caller (Extended Data Fig. 7b). Human mitochondrial reads from the shotgun nanopore sequencing were also assessed for confirmed mitochondrial pathogenicity-associated alleles (MitoTip27 and ClinGen28); we detected seven mitochondrial mutations (six from water eDNA and one from air eDNA) associated with a range of diseases, including autism, diabetes, eye diseases and cardiac diseases (Supplementary Table 6).
Fig. 4. Mutation and genetic ancestry analysis of shotgun and exome-enriched eDNA samples.
a, Known human genomic deletions (gnomAD database) detected in a nanopore shotgun water sample, including the number and size of deletions (left) and the genomic position of each deletion (right); deletion locations are denoted by blue shading. The full details of the detected deletions can be found in Supplementary Table 5. b, Percentage of sequenced reads from eDNA metagenomic samples that aligned (minimap2) to the human genome, compared with all reads generated for each sample (regardless of species of origin). Nanopore shotgun sequencing is compared with Illumina exome enrichment. Note that the same male footprint sand eDNA sample and the same high-human water eDNA sample was used for both shotgun sequencing (nanopore) and exome enrichment (Illumina). c, Haplogroup and haplotype analysis of human-mitochondrial-aligning reads for each sequenced (shotgun or exome-enriched) intentional human sample and for the water and sand bycatch Illumina shotgun-sequenced samples (sea turtle focused) with the highest number of human-aligning reads. Pie charts within the same box are from sequencing data for which the same original sample was used (that is, for shotgun and exome enrichment). d, Quantification of green sea turtle and sea turtle herpesvirus (ChHV5) and papillomavirus (CmPV1) aligning reads (minimap2) from the same room-air eDNA nanopore shotgun sequencing data as were used for identifying human-aligning reads (Fig. 3a).
Extended Data Fig. 7. Additional nanopore deletion and structural variant analysis, and Illumina exome enrichment characterisation.
(7a) Prominent human cancer-associated genes with detections within or adjacent to the gene detected (gnomAD database) detected in Moultrie Creek B nanopore shotgun water sample. Deletion location denoted by blue shading. Full details of all detected deletions can be found in Supplemental Table 5. (7b) Structural variant types identified in nanopore eDNA sequence data identified by the EPI2ME sniffles-based structural variant caller. (7c) Illumina exome enrichment versus shotgun nanopore sequencing data. Left: Total number of DNA bases mapped to the human genome (minimap2) for all exome samples, including negative field controls (Moultrie creek negative field control water eDNA and Rattlesnake Island site 2 sample 1 [no human site] sand eDNA). Right: Total number of DNA bases mapped to the human genome (minimap2) for all human exome eDNA samples, and the corresponding shotgun nanopore data (where the sample was sequenced with both approaches). Note, information is in bases not in reads because bases account for variable read length between nanopore and Illumina sequencing. For mapped read and mapped base information for all analysed samples see Supplemental Table 4.
We next assessed the feasibility of human exome enrichment on water and sand eDNA samples. Five eDNA samples were human exome enriched and then sequenced on an Illumina NovaSeq6000. Three of these samples had also been subjected to long-read sequencing (Supplementary Table 4). Despite exome enrichment, the negative field control samples (NFC sand eDNA Rattlesnake Island site 2 sample 1 and NFC Moultrie Creek) generated very few human-aligning reads (Extended Data Fig. 7c and Supplementary Table 4). For all three human-positive eDNA samples sequenced, exome enrichment increased the proportion of human-aligning reads (Fig. 4b and Extended Data Fig. 7c). Post-exome enrichment, the water eDNA sample had 48% of sequenced reads aligning to the human genome (20.72 billion human-aligning bases), representing 473× coverage of the targeted human exome regions (Fig. 4b and Supplementary Table 4).
We then examined the human population-genomic-level resolution present within our eDNA samples. The detected deletions themselves (Fig. 4a) occur at varying allele frequencies in different human populations (Supplementary Table 5). We also conducted haplogroup and haplotype analysis on all sequenced intentional human-eDNA-positive samples: four nanopore samples, three exome-enriched samples and the wildlife-focused sand and water Illumina sequencing with the highest number of human-aligning reads. Every eDNA sample assessed enabled population genomic analysis, with proportions of specific haplogroups and haplotypes varying between samples (Fig. 4c). Generally, haplotypes from European–Indian ancestry, particularly H2a2a1, were the most widely recovered from each substrate type (Fig. 4c). For samples with known participants (footprint and room air), the haplotyping matched the participant profile, while for the water eDNA samples, the results broadly matched the demographics of the area from which the sample was taken (https://www.census.gov/quickfacts/fact/table/staugustinesouthcdpflorida,staugustinecityflorida/PST045221). Even with no prior enrichment, reads containing information on genetic ancestry and parental origin have been recovered (Fig. 4c and Supplementary Table 5).
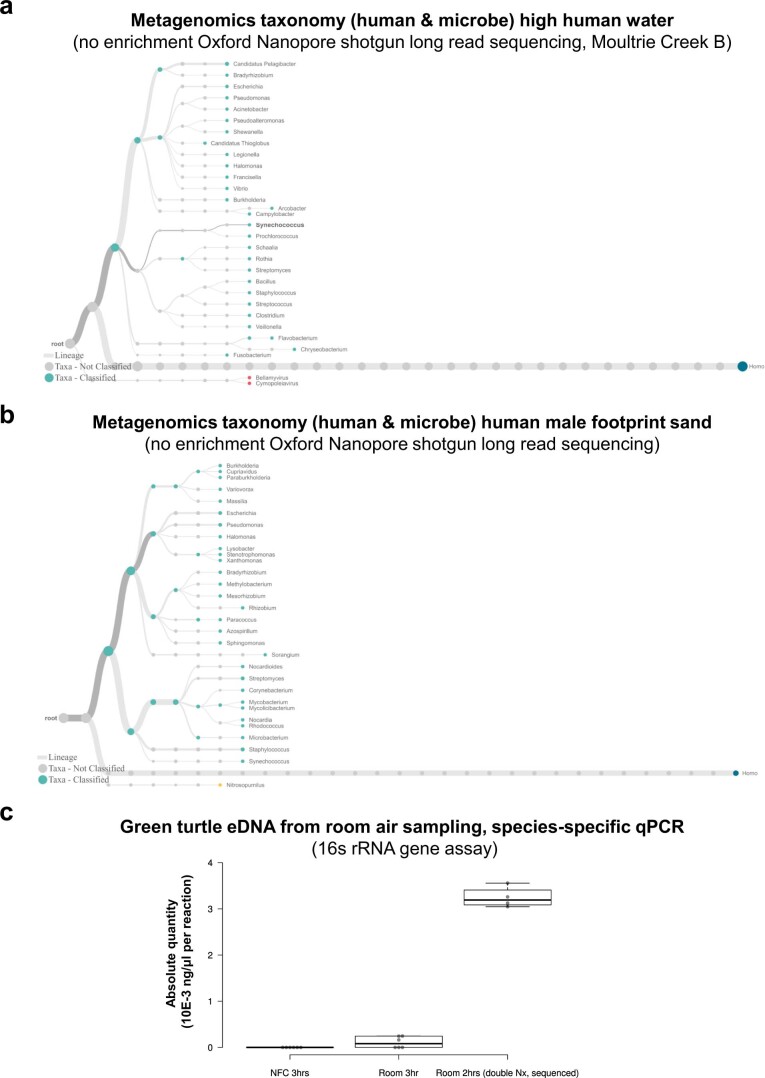
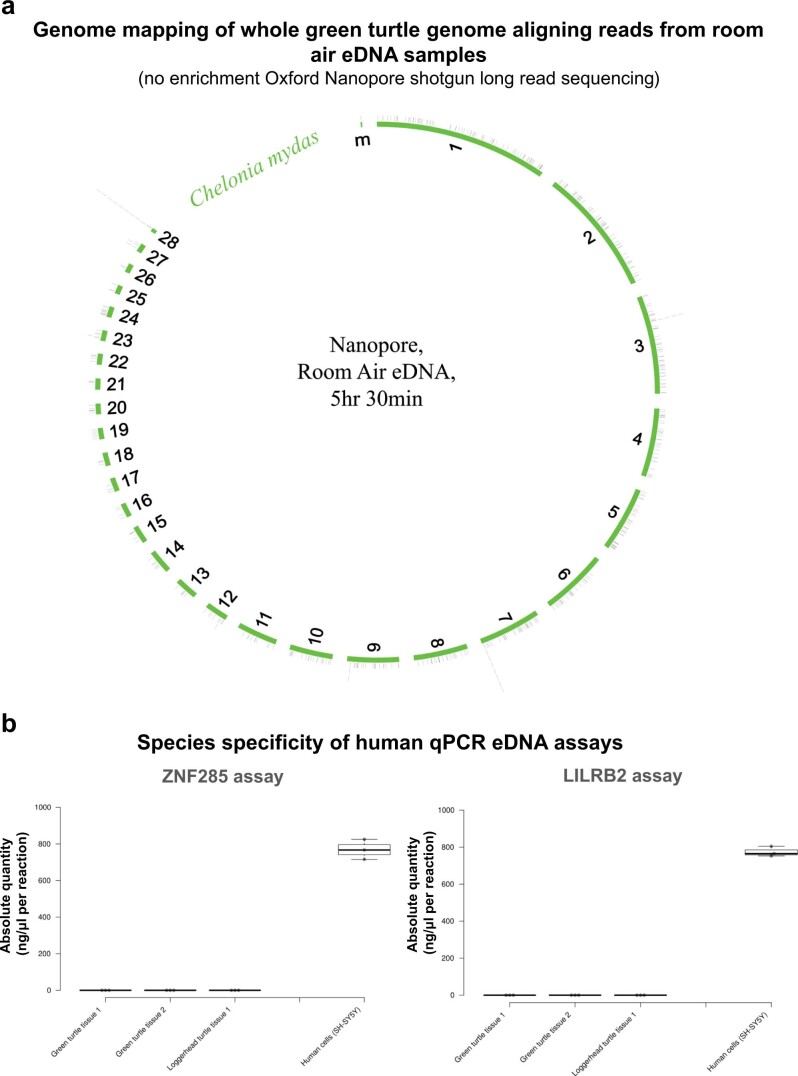
Each sample also contained a complex mix of microbial reads, demonstrating the utility of eDNA (including air eDNA) to simultaneously detect humans, animals and microbes (Extended Data Fig. 8a,b). This included the room-air sample (from a sea turtle hospital), which had airborne pathogenic (tumour-implicated) sea turtle herpesvirus and papillomavirus DNA as well as sea turtle DNA (Fig. 4d and Extended Data Figs. 8c and 9a).
Extended Data Fig. 8. Metagenomic analysis of nanopore data and qPCR-based green sea turtle species-specific qPCR-based quantification from room air eDNA samples.
(8a) Metagenomic taxonomy of microbial and human aligning reads from the high human site eDNA water sample (Moultrie Creek B), inclusion cut-off on tree, each branch contains at least 0.01% of reads. (8b) Metagenomic taxonomy of microbial and human aligning reads from the human footprint sand eDNA sample (human male footprint 4), inclusion cut-off on tree, each branch contains at least 0.01% of reads. (8c) qPCR-based species-specific quantification of green sea turtle (C. mydas) eDNA from room air samples. Absolute quantity (10E-3 pg/μl per reaction) of green sea turtle eDNA per sample. A 499 bp synthetic gene fragment was used for standard curve generation. Each qPCR reaction is a 10 μl reaction containing 1 μl of extracted eDNA template.
Extended Data Fig. 9. Green sea turtle genome coverage map from room air eDNA, and human qPCR assay cross-reactivity qPCR.
(9a) Green sea turtle (C. mydas) genome alignment map of the green turtle aligning reads (minimap2) from the 5 hr 30 min room air eDNA sample. (9b) Human eDNA qPCR assays cross-reactivity test with sea turtle (loggerhead and green) tissue samples. Absolute quantity of human genomic DNA present (ng/μl per reaction). Each qPCR reaction is a 10 μl reaction containing 1 μl of extracted genomic DNA template. Note that both assays were species-specific with no cross-reactivity (no amplification) in either sea turtle tissue sample. Loggerhead and green sea turtles can be added to the list of 27 other species (from mice to plants, see methods section) experimentally validated with which these human assays do not cross-react.
Discussion
The rapid expansion of the field of eDNA coupled with increasingly cost-effective deep-sequencing technologies promises an exponential increase in the generation of shotgun data from environmental samples over the coming years1,16,19,29,30. This heightens the likelihood of unintended large-scale HGB from the general public in eDNA studies. In the present study, we report that HGB was found in all field eDNA samples. These samples had been collected primarily for the detection of non-human species, marine turtles, animal pathogens and metagenomics8,17. With no human enrichment prior to shotgun sequencing and with sampling having been conducted in areas of relatively low human habitation densities, we nevertheless inadvertently captured a substantial amount of human genomic data. There is likely to be a continuum across which HGB may be beneficial, neutral or exploitative—for example, samples collected from a popular tourist beach are likely to be less ethically concerning than wastewater monitoring of small, defined, stable populations. Similarly, areas with greater human population numbers are more likely to return more human data. When the sites were intentionally tested, far more human eDNA was indeed recovered from aquatic sampling sites in which water had passed through populated areas. Furthermore, targeted sequencing of human eDNA from complex metagenomic samples was readily achievable with existing exome enrichment approaches. Having demonstrated the propensity of eDNA approaches to capture human eDNA equally well as that of other species, we will now consider the potential beneficial applications and ethical implications of these findings (Box 1).
At a minimum, HGB may complicate approvals for eDNA-based deep-sequencing projects, potentially requiring them to be reviewed by human-focused ethical review boards, even when humans are not the intended target. As eDNA approaches are non-invasive, limited approvals are currently needed, even when investigating endangered species. Added layers of regulation may therefore complicate the implementation and widespread adoption of eDNA approaches, hampering conservation and non-human genome research applications. The capture of non-genetic human information such as speech (eco-acoustic data) or human images (camera traps) has posed similar conundrums for conservationists as HGB. In those cases, codes of conduct, enhanced education and automated data filtering have been suggested to alleviate privacy and consent concerns31–34.
The deposition of sequencing data in a public repository is a prerequisite for scientific publication and open data initiatives. However, if eDNA sequencing datasets also include human genomic information, this may produce a potential conflict. An open ethical question is therefore whether any such data would have to be pre-filtered to remove human sequencing data prior to deposition, similar to the de-identification of patient data35. This in turn raises issues relating to transparency and the correct filtering criteria. Furthermore, with whom should the responsibility of policing such a solution lie: journals, data repositories, ethical committees or investigators themselves? Another alternative is attempting to actively block the sequencing of human DNA from eDNA samples. However, a recent study reported that even when human blockers were applied, human reads were still detected36.
Current deep-sequencing approaches suffer from a somewhat similar issue as HGB, whereby genomic information from personnel who process samples may be inadvertently sequenced37,38. However, in terms of ethical considerations, this inadvertent genome capture from contamination occurs from experienced personnel who are well versed in the technology. Environmental-DNA-based inadvertent capturing of genomic information from the general public is a more complex ethical conundrum, as the genomic information captured comes from individuals mostly unaware of the technology use and oblivious to the fact that their genetic information has been inadvertently obtained. Complicating matters, researchers are already exploring the potential of eDNA for individual-level identification and tracking in wildlife populations39–41. HGB in eDNA metagenomics studies therefore raises the intriguing question of whether individual humans could also be identifiable from eDNA data; this is likely, given the unfragmented nature of eDNA reported here. This could require eDNA studies to obtain prior informed consent, a near-impossible task. Given the large geographical range over which eDNA can travel1,6,42,43 (particularly for aquatic samples44–46), it is impractical to imagine that prior informed consent could realistically be obtained. Even with no targeting or enrichment, our MinION shotgun sequencing was able to identify the genetic ancestry within pooled human populations and to identify variants associated with disease susceptibility. The long eDNA reads sequenced here suggest that with targeted enrichment of informative genomic locations, one could achieve individual-level identification even from pooled samples. Given the advanced targeting approaches already in existence for genomic regions of interest (for example, from disease and population genomics research, nanopore adaptive sequencing47–51 or the exome enrichment used in this study), targeting specific regions or variants from human eDNA samples is already completely feasible.
While our data demonstrate the possibility of human genomic bycatch in wildlife-oriented eDNA studies, they also highlight the feasibility of targeted human eDNA applications. Particularly sensitive or informative regions of the human genome could be intentionally targeted. Human privacy issues are particularly relevant to the increased use of pathogen detection from human wastewater during the COVID-19 pandemic1,11,12. Even in relation to pathogens, the legal and ethical implications of wastewater monitoring have not been adequately considered. Wastewater is currently utilized to detect illicit drugs, antidepressants, stress markers and alcohol consumption52. Our findings show that such ethical dilemmas need to be considered not only for wastewater sampling but for the field of eDNA as a whole, with HGB feasible from air, aquatic and substrate environmental sampling.
Given the demonstrated feasibility of human eDNA analysis, including disease-associated loci calling, it is likely that beneficial applications may exist for such human-orientated eDNA approaches (Box 2). For example, one could utilize wastewater or air eDNA-based sampling to correlate the level of pathogens with the abundance of susceptibility loci in a given population1,13,15,53,54. This may be especially feasible given the ever-increasing portability of DNA sequencing/surveillance technology1,29. It is also immediately feasible to consider intentional human and pathogen eDNA sampling from specific sources, such as substrate footprints or filtered-air eDNA (as we report here) from specific locations—for example, rooms in the home, public indoor or outdoor spaces, hospital wards, or even special diagnostic rooms designed to filter the host and pathogen eDNA shed by a single patient over a short period. Here we report that room-air sampling in a clinical setting (a veterinary hospital) recovered host (animal patient), human (staff) and vertebrate viral pathogens, suggesting future novel monitoring approaches in human and animal medical settings and beyond. Alternatively, water eDNA collected before wastewater leaves the home could be utilized, linking to continual health monitoring and chronic disease management initiatives55. This is particularly pertinent to routine continual health monitoring for somatic mutations, which arise later in life and can be drivers of life-threatening diseases such as cancer. Human eDNA approaches can be combined with advances in the ongoing genomic medicine revolution24 to enable novel applications, as evidenced here by the ability to detect specific human disease-associated mutations from eDNA and to enrich for human genomic regions of interest (for example, exome) and simultaneously detect microbes, including pathogenic viruses.
Haplotyping and phylogenetic analysis of individuals (whale sharks, sea turtles and kakapo) from eDNA samples have already been achieved, although to date these have tended to be samples dominated by a single individual17,47,51,56. Our study adds humans to this list and highlights how haplotyping can be achieved from complex multi-individual (metagenomic) samples with diverse population structures. Given its less biased nature, human eDNA may also help redress the balance in relation to the lack of sequenced genomes from diverse populations and underrepresented rare human alleles in genomic databases. Traditionally, these databases have been skewed towards populations of European ancestry57.
Separately, genetic analysis is already being applied to combat the illegal wildlife trade by identifying the source animal populations58,59. Similarly, it may be possible to adopt such approaches in targeting human eDNA recovered from illegal wildlife parts (poacher/trader handling) to broadly identify the geographic human populations through which the illegal parts transited. Human eDNA could also be employed to identify sites of archaeological importance; such possibilities include sampling of remote stagnant waterbodies (for example, bogs) to identify undiscovered sacrificial sites, or eDNA sampling to help in the recovery of more recent human remains. Even search-and-rescue missions could use eDNA techniques if currently in-trial air eDNA sampling by drones is successful. Recently, it has been postulated that human eDNA extracted from the air could also have novel forensic and criminal investigation applications60–62. Our human population genomics assessment of airborne DNA is an early step in this direction, providing proof-of-concept of the recovery of shotgun-sequencing-quality human DNA from room air. Both ancient and contemporary DNA have their own established ethical procedures, although in those disciplines too, the pace of advancement has outpaced the dialogue about research ethics63–66. Vertebrate air eDNA is a recent field of study, and improved capture devices is an active area of research from which human airborne DNA applications could benefit36. Water-based eDNA approaches have also been postulated to be beneficial for forensic investigations67,68. Forensic science has well-established ethics procedures, although issues pertaining to racial discrimination persit69,70. Taken together, forensic applications can be envisaged for air, soil and water eDNA approaches, especially in light of the intact non-fragmented nature of eDNA reported here.
While benefits of human eDNA analysis may exist, there could conceivably be more worrying applications of such technology. The accumulation of population-level genomic databases is currently highly desirable, being a valuable research and commercial commodity71,72. While some projects maintain the genomic data and subsequent findings in public ownership, the lucrative economic potential of such databases means that a host of private companies have also been rapidly accumulating them (for example, DNA ancestry companies and genomic medicine companies) and selling access72,73. Countries and corporate entities are racing to create ever-larger pan-genomic patient/population datasets. Examples include the 100,000 Genomes Project and the new Genome UK Strategy (https://www.gov.uk/government/publications/genome-uk-the-future-of-healthcare); the Biobank project, which aims to sequence half a million genomes (https://www.sanger.ac.uk/collaboration/uk-biobank-whole-genome-sequencing-project/); and the National Institutes of Health All of Us Research Program (https://allofus.nih.gov/), a project to sequence the genomes of one million US citizens. Such pan-genomic activities have the potential for great public good, including advanced medical and pharmaceutical applications, but they have been an ethical minefield regarding ownership, data protection, insurance coverage and privacy issues24,72,74–76. Examples from digital databases also clearly show that wherever valuable databases exist, there is an ever-present temptation to exploit the data for financial gain, whether legally or otherwise. The Cambridge Analytica scandal, which involved complex harvesting of data from Facebook profiles77, is one such example, and Google’s harvesting of medical records with individual identifying information still intact is another78.
Human eDNA applications present similar concerns: they could be employed for the surveillance of individuals, minority groups (genetic ancestry) or genetically driven disabilities, or to obtain genomic information from local populations without their knowledge or consent, including from ‘valuable’ genetically diverse indigenous populations (uncontacted tribes, particular ethnic groups and so on). Such scenarios expand the current issues regarding the commodification of genomic information, which is already a particularly acute concern in relation to indigenous peoples72,79–81. These novel human eDNA applications may also enable unscrupulous (though possibly not technically illegal) entities to perform covert mass capture of genomic data from populations, to further expand population-level genomic databases. Furthermore, such resources can then be utilized for enhanced monitoring of individuals for less savoury purposes. Genetic/genomic surveillance is a serious ethical concern, with documented human rights abuses having already occurred whereby national DNA databases were used with other surveillance data to monitor minority populations70,82. The application of human eDNA approaches could further undermine genetic consent, limiting the ability of threatened minorities to withhold their genetic information. In the future, human eDNA could also be utilized to determine whether members of a genetically distinct group were present in a given population—for example, through wastewater monitoring or air filtering at checkpoints, in urban areas or in private dwellings. Such potential is particularly chilling given the propensity of humans to carry out ethnic persecution and genocide throughout our history.
Many of the ethical issues raised here are more likely to arise when sampling sites are closer to human urban areas. Similarly, issues pertaining to individuals or ethnic groups are more likely to be pertinent in areas with small, stable populations of humans contributing to the recovered eDNA—for example, a specific watershed or portion of a sewage system. It is clear that in the short term the convergence of massively powerful deep-sequencing technologies with improvements in ecological eDNA sample capture approaches raises serious questions about human genomic bycatch, requiring discussion and regulatory consideration from scientific, ethical, regulatory and social perspectives. Regulators, researchers, funders and other stakeholders should develop responses to the ethical implications of HGB and intentional human eDNA applications. Such planning should be initiated immediately, pre-empting the technology becoming even more widespread, affordable and entrenched, be it for beneficial or exploitative applications. Such ex ante planning is crucial for ensuring that laws and ethics stay ahead of emerging technology. Conversely, these same eDNA approaches can open up novel beneficial applications in areas from human health to criminal forensics.
Box 1 Potential problematic implications of the capture of human genomic eDNA data.
Unintended consequences:
Requirement of human-study-related ethical approvals for wildlife studies
Lack of human subject consent/breach of privacy
Public deposition of eDNA data including human genomic data
Inadvertent location tracking
Inadvertent genome harvesting
Potential malicious applications:
Genome harvesting—the ability to illegally/unethically harvest human genomic data from local populations/ethnic groups without their knowledge or consent
Covert accumulation of human genetic data for malicious or commercial purposes (for example, genomic surveillance or big-data-fuelled discovery)
Genetic surveillance—individual tracking (similar to forensics/wildlife applications)
Genetic surveillance—unethical tracking/locating of ethnic groups/populations
Genetic surveillance—potential for involuntarily genetic surveillance from investigative applications, including the recovery of bystander shed genetic information or intentional overreach
Bio-piracy of human genetic data from populations and countries (akin to flora/fauna genetic bio-piracy)
Box 2 Potential beneficial applications of human eDNA as the nascent field matures.
The discovery of novel human genetic variation can help redress the historical imbalance in human genomic databases not spanning the range of human diversity.
Population-based disease risk susceptibility studies can be carried out, particularly wastewater- or air-based when coupled with active pathogen surveillance.
Non-invasive monitoring of host genetics, pathogen load and transmission studies can be conducted, including from pooled settings (including being able to disentangle the number of pooled individuals contributing to non-controlled-environment samples).
Human eDNA can be a new tool in continual health monitoring and continual personalized medicine biomarker monitoring for chronic disease management initiatives (particularly pertinent to somatic mutations, which arise spontaneously and can be drivers of life-threatening diseases such as cancer).
It can enable sensitive quantification and source identification of human effluent entering and polluting waterways and aquifers (from septic tank leaching and release of improperly treated wastewater).
Forensic and criminal investigative applications can aid in solving crime. Air eDNA holds particular novel promise.
Human eDNA can assist in the recovery of missing persons/deceased remains (particularly drone-enabled air eDNA in remote locations, especially if coupled with real-time eDNA detection technology and remote reporting).
It can help locate sites of archaeological importance (cryptic human remains, such as sacrificial sites in remote bogs).
It can serve as a roadmap for future wildlife (fauna and flora) eDNA studies. Trialling approaches and tools with human eDNA and existing rich human genomic databases will be highly informative for what can be achieved for wildlife eDNA population genomics (haplotyping to individual, disease risk and so forth) once sufficient wildlife genomic resources/databases are established.
Methods
Sample collection, DNA extraction and sequencing
All laboratory procedures from sampling through final analysis (sequencing or qPCR) were conducted in a way that minimized any human DNA contamination from investigators. Although the Illumina HiSeq eDNA samples had also been sampled and utilized for sea turtle and pathogen research, the original study design for these samples included assessment of the human-aligning reads from these shotgun data. Therefore, all appropriate precautions to avoid contamination were taken. This included no contact of investigators or samplers with the substrates being sampled (water or sand), new nitrile gloves being used for each sample collection, frequent glove changes throughout sample processing, cleaning of equipment and benchtops with bleach prior to use, and negative field controls being treated identically to genuine field samples throughout all processes from extraction through qPCR/sequencing (see below). All eDNA samples were extracted in labs that do not process human samples: a sea turtle lab in Florida and a chick/mouse developmental biology lab in Ireland. The Irish qPCRs were set up in a laminar flow cabinet after a protracted 1 h exposure of UV of the cabinet and equipment. It was originally postulated that the sea turtle rehabilitation tank water sample (2017, HiSeq) would contain more human DNA than the field water samples, due to hospitals staffs’ interactions with the tank water and sea turtle patients. For all subsequent eDNA sampling, we had already established that the 2017 tank sample contained less human eDNA than the 2017 field samples. We therefore continued to observe strict procedures to avoid investigator contamination in all subsequent sampling in order to be able to investigate HGB. While we intended in advance to assess the level of human eDNA recovered from the 2017 and 2020 samples, no human factors (such as population density) were considered when selecting sampling sites. Site selection was purely based on the wildlife eDNA considerations of these sites—that is, site selection was not biased towards areas with high human densities. Conversely, site selection for all 2022 samples was intentionally directed towards sites with high or low human perturbations (primarily based on human population density).
Previously extracted8 green and loggerhead sea turtle DNA from tissue samples was used for species specificity qPCR tests. DNA was extracted from tissue using a Qiagen DNeasy Blood and Tissue kit (Qiagen, cat. no. 69504) according to the manufacturer’s instructions. SH-SY5Y cells (ATCC, cat. no. CRL-2266) were gifted by the Loesgen lab (University of Florida), and no human cells were processed in the same lab as the eDNA samples. DNA was extracted using a Qiagen DNeasy Blood and Tissue kit according to the manufacturer’s instructions. SH-SY5Y genomic DNA was used for generating standard curves. To avoid any potential contamination, the standard curves were run only after all eDNA qPCRs had been completed.
HGB samples
Seawater (rehabilitation tank and oceanic) and beach sand eDNA samples were collected between 2017 and 2021 and sequenced as part of our sea turtle and sea turtle pathogen research8,17. All samples originated from Florida, US8,17. The tank sample is a pooled8,17 sample containing eDNA extracted from seawater from five rehabilitation tanks at the University of Florida’s Whitney Laboratory for Marine Bioscience and Sea Turtle Hospital. The four main tanks housed juvenile green sea turtles and were 240 cm in diameter and had a full volume of 2,270 l, and a smaller loggerhead post-hatchling tank had a volume of 480.75 l. The separately extracted eDNA from each tank was pooled in equal volumes prior to library preparation.
Intentional human samples
River, estuarine, seawater (oceanic) and beach sand samples were collected between May and July 2022 (Supplementary Table 2). Negative field control samples of water and sand were also collected and processed as per the study samples. For negative field control water sampling, 1 l of MilliQ water (Florida) or 1 l of Qiagen Nuclease-free water (Ireland, cat. no. 129117) was transported from the laboratory to the rehabilitation or wild sampling locations and stored in a cool box with the environmental samples to monitor for potential contamination during sampling, transportation and processing. For sea turtle negative field control sand sampling, 50 ml of beach sand was collected away from suspected turtle presence (that is, away from sea turtle tracks or obvious human activity) on each sampling trip. For human negative field control sand sampling, 50 ml of beach sand was collected away from suspected human activity (such as footprints) on each sampling trip and from a restricted-access location on Rattlesnake Island, part of the Fort Matanzas National Monument managed by the US National Park Service. The water and sand negative field controls were filtered and extracted alongside the other collected sand and water samples from each sampling trip and subjected to the same next-generation sequencing conditions and qPCR conditions (intentional human samples). The standard volume of seawater filtered (0.22 µm pore Millipore Sterivex-GP Pressure Filter Units (Merk Millipore, cat. no. SVGPL10RC)) was 500 ml for each DNA sample8,17. Samples of less than 500 ml (Supplementary Tables 1 and 3) were a lower volume due to debris clogging the filter and preventing larger volumes being filtered. One sample (‘tidal pool’) had 1 l of seawater filtered. For sand eDNA, a 50 ml tube was filled with sand from each sampling event, with 10 ml of this sand used per individual eDNA extraction17. Human-present air samples were collected from a 280 ft2 room while the participants went about their daily work activities (that is, they could enter and exit the room throughout the sampling period), with a maximum of the same six participants using the room for a portion of the sampling period. The room was air-conditioned (outside air) and had an external door that was opened and closed, and occasionally left open for certain work procedures. Negative field control samples of air were also collected and processed as per the study samples. For air eDNA, two types of negative field controls were collected: (1) a filter kept in the room being sampled for the duration of sampling, but with no air being pulled through it (that is, no pump), and (2) air filtered (with a pump) from a room with no humans present at the time of filtering or during the previous 24 h. Human-related sampling was conducted with University of Florida Institutional Review Board (IRB-01) ethical approval under project number IRB202201336, with all participants providing informed consent. Four participants (three female and one male) provided sand footprint samples, and six participants (five female and one male) provided room-air samples (pooled room air). Participation was on a voluntary basis, and the participants received no compensation.
Prior to filtration and between every sample, the laboratory surfaces and filtration equipment (all standard laboratory equipment involved in the washing and filtration process, as well as the filtration pump itself) were disinfected with 70% ethanol, and the sampling equipment (collection bottles) was disinfected (washed thoroughly) with 10% bleach and rinsed thoroughly with deionized water.
Sand
1X TE—IDTE pH 8.0 1X TE Solution (Integrated DNA Technologies, cat. no. 11-05-01-09) was added to each individual sand sample in individual 50 ml Falcon conical centrifuge tubes, at approximately two times the volume of sand (10 ml of sand and 20 ml of 1X TE). The samples were shaken gently by hand and then set on a rocking platform for 1 h at room temperature, with additional gentle shaking by hand every 15 min. The samples were rested until sand had sunk to the bottom of each tube; then, the supernatant was immediately pipetted into a 60 ml sterile BD luer lock syringe (Fisher Scientific, cat. no. 136898). The samples were then hand-filtered using 60 ml BD luer lock syringes through 0.22 µm Sterivex-GP Pressure Filter Units (Millipore, cat. no. SVGPL10RC) and capped with B.Braun luer lock caps (Medline, cat. no. BMGTMR2000B). Finally, 740 µl of Buffer ATL and 60 µl of Proteinase K from a Qiagen DNeasy Blood and Tissue Kit (Qiagen, cat. no. 69504) were added to each sample, and they were placed in 50 ml Falcon conical centrifuge tubes in a rolling incubator overnight (24–26 h) at 56 °C.
Water
The water samples were pumped (by hand (in Ireland) or electronically (in Florida)) through 0.22 µm Sterivex-GP Pressure Filter Units (Millipore, cat. no. SVGPL10RC) and capped with B.Braun luer lock caps (Medline, cat. no. BMGTMR2000B). Hand-pumping was with sterile 60 ml BD luer lock syringes. Electronic pumping was done using a GeoTech Peristaltic Pump Series II. Then, 740 µl of Buffer ATL and 60 µl of Proteinase K from a Qiagen DNeasy Blood and Tissue Kit were added to each sample, and they were placed in 50 ml Falcon conical centrifuge tubes in a rolling incubator overnight (24–26 h) at 56 °C.
Air
For air eDNA sampling, room air was passed through 0.22 µm pore Millipore Sterivex-GP Pressure Filter Units (Merk Millipore, cat. no. SVGPL10RC), using a Welch vacuum pump (2019LD-4112) or a GeoTech Peristaltic Pump Series II. Environmental DNA was extracted8,17 as for the water and sand samples, except that only 20 μl of Proteinase K was added per filter and the 56 °C ATL Buffer and Proteinase K incubation was conducted for 1 h.
DNA extraction and sequencing
For all three sample types (sand, water and air), after the 56 °C incubation, the solutions of Buffer ATL (after water or air filtration, or after sand washing and filtration), Proteinase K and eDNA were transferred from the Sterivex-GP Pressure Filter Units to 2 ml microcentrifuge tubes using 10 ml BD Slip Tip Sterile Syringes (Fisher Scientific, cat. no. 14823434). DNA was then isolated using a modified Qiagen DNeasy Blood and Tissue Kit protocol8,17,83. Following incubation, equal volumes of 800 µl (sand and water) of AL Buffer and 800 µl (sand and water) of ice-cold ethanol were added to each sample, and they were vortexed vigorously and microcentrifuged after each addition. For the air samples, 400–500 µl of each solution was used (as a lower volume of ATL solution is recovered from the initially dry Sterivex filters). Each sample was loaded into a DNeasy spin column and centrifuged at 6,000g for 1 min, with flow-through being discarded after each spin (repeated until the entire contents of each sample were spun through the spin column). Then, 500 µl of Buffer AW1 was added, and the samples were centrifuged at 6,000g for 1 min. Next, 500 µl of Buffer AW2 was added, the samples were centrifuged at 16,000g for 3 min, flow-through was removed and they were spun for an additional 1 min at 16,000g. DNA was eluted with 70 µl of AE Buffer (incubated at 70 °C before being added to the spin column), incubated on the column at room temperature for 7 min and centrifuged into a 1.5 ml microcentrifuge tube at 6,000g for 1 min. DNA concentration was measured on a ThermoScientific Nanodrop 2000 Spectrophotometer (Fisher Scientific), and the samples were stored at −20 °C until qPCR or shotgun sequencing.
Library preparation and Illumina shotgun sequencing were conducted at the University of Florida’s Interdisciplinary Center for Biotechnology Research Core Facilities. Four water eDNA samples (2017) were sequenced on an Illumina HiSeq 3000, and all subsequent Illumina samples (seven water and ten sand eDNA) were sequenced on an Illumina NovaSeq 6000 (Supplementary Table 1)8,17. All Oxford Nanopore samples were sequenced on a MinION in the Duffy lab at the University of Florida’s Whitney Laboratory for Marine Bioscience. This device was not previously used for any human samples. We used a personal MinION sequencer instead of the previous core facility high-throughput Illumina sequencer as there were some human-aligning reads in our Illumina negative field control sample, although these were 13 to 38 times fewer human reads than were recovered from environmentally derived eDNA samples (Supplementary Table 1). MinION libraries were prepared according to the manufacturer’s instructions, using the following kits: Oxford Nanopore Technologies (ONT) Ligation Sequencing Kit (SQK-LSK110, cat. no. 76487-106), NEBNext Companion Module for ONT Ligation Sequencing Kit (cat. no. E7180S) and ONT Flow Cell Wash Kit (EXP-WSH004, cat. no. 76487-116). They were then sequenced on ONT Minion Flow Cells (cat. no. 76487-106). For run times and the percentage of pores available, see Supplementary Table 3. All sea-turtle-related sequenced samples including raw reads are deposited in the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/) under BioProject ID PRJNA449022. All human-eDNA-related sequenced samples are under BioProject ID PRJNA874696.
Exome capture libraries were constructed and sequenced at the UF ICBR Gene Expression and NextGen Sequencing Cores. Five water or sand eDNA samples (three intentional human-centred samples and two negative field controls) were used for Illumina DNA Prep with Enrichment, Exome Panel (cat. no. 20020183, captures 45 Mb of exonic content) exome capture analysis, according to the manufacturer’s instructions. Briefly, eDNA was fragmentized, adapters ligated and amplified for nine cycles and purified with Ampure XP beads (Beckman Coulter, cat. no. A63881). The five samples were pooled with equal volume for one exome hybridization according to the user guide, and the probe hybridization was performed at 95 °C for 5 min, one cycle of 1 min each, starting at 94 °C for the first cycle, then decreasing 2 °C per cycle for 18 cycles, and a hold for 90 min at the final temperature. After the exome hybridization, the probe capture, wash and elute was performed, followed by library enrichment with ten cycles of PCR amplification. Exome sequencing was conducted on an Illumina NovaSeq 6000 S4 flow cell for 2× 150 bp cycles aiming for 50 million reads per sample (with negative field controls expected to return fewer reads due to a lack of human eDNA).
Bioinformatic analysis
All bioinformatic tools were utilized using the default parameters, unless otherwise stated. The Galaxy platform (https://usegalaxy.eu/) was used for bioinformatic analysis84,85, with NanoGalaxy also used for nanopore sequenced data86. All samples were checked for quality (FastQC, v.0.73; ref. 87), adapters and low-quality reads were trimmed (<20 quality score) (Trim Galore! (https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) v.0.6.7 for HiSeq and NovaSeq data; Porechop v.0.2.4 (ref. 88) for Oxford Nanopore sequence data), and high-quality reads were aligned (Bowtie2, v.2.4.2; ref. 89) to the human reference genome (Hg38, https://www.ncbi.nlm.nih.gov/genome/?term=human%20genome%2038) (paired-read alignments, Illumina and single-read alignments, nanopore) and aligned (minimap2, v.2.24; ref. 90) to the newly released complete human genome (T2T-CHM13v1.1; ref. 91). Trimmed air eDNA nanopore data were also aligned (minimap2, v.2.24; ref. 90) to the following reference genomes: green sea turtle (Chelonia mydas) (rCheMyd1, NCBI accession number: GCF_015237465.2 (refs. 92)), ChHV5 (NCBI accession number: HQ878327.2 (ref. 93)) and CmPV1 (NCBI accession numbers: MT179559.1, MT179558.1 and EU493091.1)94,95. To examine human genetic reads in the water and sand samples, StringTie (v.2.1.7; ref. 96) was used to identify reads aligning to the human Y chromosome only, by collating Y-chromosome-specific reads (gene abundance per sample abundance). Total reads per sample aligning to the human Y chromosome were also quantified using Samtools idxstats (v.2.0.4; ref. 97), using alignment files as input. The human Y chromosome was selected because it is fast-evolving and can therefore confirm the presence of genuine human reads25. Total reads per sample aligning to human nuclear and mitochondrial regions were also quantified using Samtools idxstats (v.2.0.4), using alignment files as input. Human mitochondrial haplogroups were classified in MitoMaster98, using Haplogrep99 and Phylotree 17 (ref. 100), with the pathogenicity of mitochondrial variants determined by MitoTip27 and ClinGen28. Human mitochondrial haplogroup charts were produced in RStudio101, using the webr package v.0.1.5 (https://github.com/cardiomoon/webr), and the human genome coverage plots were produced using the ggplot package v.3.4.0 (ref. 102).
The ONT EPI2ME platform was used for structural variant calling and metagenomics, and adapter and low-quality read trimming was conducted with Porechop. EPI2ME Structural Variant Caller was run using the default parameters (including a minimum of three reads to support each call), with the only exception being a minimum structural variant length of 20 bp. Alignment was to the human reference genome (GRCh38; ref. 103) using minimap2 (ref. 90), and structural variant calling was performed with Sniffles104,105. Metagenomic analysis was conducted with What’s in My Pot (v.2021.11.26), which utilizes Centrifuge and Dustmaker106–108.
For the structural variant analyses on ONT sequencing, we used minimap v.2.17-r941 (ref. 90) to align adapter pre-trimmed nanopore eDNA sequencing reads against the human genome GRCh37 with the default parameters. The resulting files were indexed, binarized and sorted using samtools v.1.10 (ref. 97), followed by mapping coverage estimation with samtools coverage. For high-sensitivity rearrangement calling, Sniffles v.2.0.7 (refs. 104,105) was used to detect structural variants in each sample, using the specific settings ‘non-germline’ and ‘minsupport = 1’ to increase sensitivity to single split-read resolution. The results of individual runs were used as inputs for a second, combined rearrangement calling by Sniffles’ multi-sample input support. To screen for known human deletions, outputs were processed using the vcfR (v.1.12.0) library in R109. Deletion call regions were matched against the gnomAD v.2.1 structural variants database26, using a lenient distance threshold of up to 5% of the total event length around both the 5′ and 3′ deletion break-end positions. We manually verified the resulting copy number variants’ ONT sequencing support using IGV110. Deletion hits were visualized on GRCh37 chromosome maps using the chromoMap (v.4.1.1) library in R111. Functional clustering of deletion gene hits was performed using HumanBase (https://humanbase.net/) functional gene network analysis112, accessed 21 October 2022.
Quantitative PCR
Two human Applied Biosystem pre-validated Taqman Gene Expression qPCR assays directed against the LILRB2 gene and the ZNF285 gene (assay IDs Hs01629548_s1 and Hs00603276_s1, respectively) were selected for use as species-specific human assays, on the basis of having no cross reactivity with over 27 other species from mice to plants (https://www.thermofisher.com/order/genome-database/; mouse, rat, Arabidopsis, C. elegans, fruit fly, bovine, dog, Chinese hamster, goat, white-tufted-ear marmoset, guinea pig, zebrafish, horse, chicken, soybean, cynomolgus monkey, sheep, rabbit, rice, rhesus monkey, baker’s yeast, fission yeast, pig, bread wheat, wine grape, western clawed frog and maize) and having both primers and probe within a single exon (that is, detect DNA). We also showed that these assays did not cross-react with green sea turtle or loggerhead sea turtle DNA (Extended Data Fig. 9b). A pan-eukaryotic 18S rRNA gene (Applied Biosystem, 4352930E) pre-validated Taqman Gene Expression assay, which also has both primers and probe within a single exon (that is, detects DNA), was used to quantify the total level of pan-eukaryotic DNA in each of the Irish samples. Green sea turtle species-specific qPCR from air eDNA was conducted as previously developed and validated assays targeting the 16S rRNA gene, including the use of a 499 bp synthetic gene fragment for standard curve generation8,17 (forward primer, TGCAAAAGCGGGAATAACAC; reverse primer, TCGCCCCAACCAAAAATATAG; FAM labelled (ZEN and Iowa Black double quenched) probe, CAACTATCTATACCCACTCACTCTAAGGACCTATAA (synthesized by Integrated DNA Technologies)). For the green sea turtle 16S rRNA synthetic gene fragment sequence, see Supplementary Table 7.
The qPCR reaction mixtures were performed on 384-well plates in a total volume of 10 μl per well: 5 μl of TaqMan Fast Advanced Master Mix (Fisher Scientific, cat. no. 4444557), 3.5 μl of nuclease-free water (Fisher Scientific), 0.5 μl of the respective assay (primer/probe mix, manufacturer-supplied concentration) and 1 μl DNA template (or, for no-template controls, an additional 1 μl of nuclease-free water per well). Depending on the sample type and the sample volume available, each biological sample was run in three to six technical replicates (three technical replicates for tissue samples and four to six technical replicates for eDNA samples). Negative field controls had the same number of technical replicates run as their corresponding eDNA samples. No template controls were run in triplicate on every qPCR plate. qPCR reactions were performed on an Applied Biosystems QuantStudio 6 Pro (Florida samples) or an Applied Biosystems QuantStudio 7 Flex (Irish samples) with the following cycling parameters: 95 °C for 20 s for one cycle, followed by 45 cycles of 95 °C for 1 s and 60 °C for 20 s. The qPCR results were plotted with BoxPlotR113 (http://shiny.chemgrid.org/boxplotr/) with every datapoint displayed. Tukey whiskers (extending to data points that are less than 1.5× the interquartile range away from the first/third quartile) were utilized for every box plot. One box is graphed per single sample, consisting of all qPCR technical replicate wells for that sample. Biological replicates are denoted by the letters A–D at the end of the sample name. Biological replicates are not pooled on any box plots; each sample is denoted by its own box.
Permitting statement
Sea-turtle-related sampling was carried out under Florida Fish and Wildlife Conservation Commission permits and with ethical approval from the University of Florida’s Institutional Animal Care and Use Committee; see ref. 17 for the full details. Human-related sampling was conducted with University of Florida Institutional Review Board (IRB-01) ethical approval under project number IRB202201336, with all voluntary participants providing informed consent. Sampling at the Fort Matanzas National Monument (Rattlesnake Island) was conducted under a United States Department of the Interior National Park Service permit, permit number FOMA-2022-SCI-0003.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Supplementary Fig. 1.
Supplementary Tables 1–7.
Acknowledgements
Funding for the initial HGB eDNA study was generously provided by the National Save the Sea Turtle Foundation under project name Fibropapillomatosis Training and Research Initiative (D.J.D.), a Welsh Government Sêr Cymru II and the European Union Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 663830-BU115 (D.J.D.). This research was also supported by Gumbo Limbo Nature Center d/b/a Friends of Gumbo Limbo (a 501c3 non-profit organization) through a generous donation through their Graduate Research Grant programme (J.A.F.) and by an Irish Research Council Government of Ireland Postgraduate Scholarship, under project no. GOIPG/2020/1056 (L.W.). Intentional human eDNA research was funded by D.J.D.’s University of Florida start-up funds. M.R.S. was supported by an EMBO long-term fellowship (ALTF 544-2021). We thank M. Q. Martindale, N. Condron, K. Yetsko, S. Creer, A. Pacetti, E. Ryan, C. Eastman and all of our generous co-authors on the wildlife and pathogen research papers for which these HGB samples were originally generated8,17. We thank P. Murphy and R. Rolfe for facilitating eDNA extraction of Irish samples in their lab in the Zoology Department, Trinity College Dublin, and A. Krstic, W. Kolch, A. G. Munoz and the Conway Core Facilities staff for facilitating qPCR of the Irish samples at Systems Biology Ireland and the Conway Institute of Biomolecular and Biomedical Research at University College Dublin. We also thank F. Duffy and I. Duffy for assistance with sampling; M. ten Cate and A. Whilde for gifting supplies; K. Foote, A. Rich and the NPS staff of the Fort Matanzas National Monument for valuable assistance with permitting and facilitating access to Rattlesnake Island and site selection; and S. Loesgen (University of Florida) for the SH-SY5Y cells. Finally, we thank the anonymous study participants who permitted us to collect their footprints and room air for human eDNA analysis, with full ethical approval and informed consent.
Extended data
Author contributions
D.J.D. designed and supervised the project. D.J.D., J.A.F., T.O., S.A.K., V.S. and J.W. conducted the sampling. J.A.F., D.J.D., S.A.K., N.M. and V.S. generated the data. L.W., M.McC., M.R.S. and D.J.D. conducted the bioinformatics analysis. All authors read and approved the final manuscript.
Peer review
Peer review information
Nature Ecology & Evolution thanks Lara Urban and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
All Illumina sequenced samples including raw reads are deposited in the NCBI database (https://www.ncbi.nlm.nih.gov/) under BioProject ID PRJNA449022. All Oxford Nanopore sequenced samples including raw reads are deposited in the NCBI database (https://www.ncbi.nlm.nih.gov/) under BioProject ID PRJNA874696.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Liam Whitmore, Mark McCauley, Jessica A. Farrell.
Extended data
is available for this paper at 10.1038/s41559-023-02056-2.
Supplementary information
The online version contains supplementary material available at 10.1038/s41559-023-02056-2.
References
- 1.Farrell JA, Whitmore L, Duffy DJ. The promise and pitfalls of environmental DNA and RNA approaches for the monitoring of human and animal pathogens from aquatic sources. Bioscience. 2021;71:609–625. doi: 10.1093/biosci/biab027. [DOI] [Google Scholar]
- 2.Adams CIM, et al. Beyond biodiversity: can environmental DNA (eDNA) cut it as a population genetics tool? Genes. 2019;10:192. doi: 10.3390/genes10030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boussarie G, et al. Environmental DNA illuminates the dark diversity of sharks. Sci. Adv. 2018;4:eaap9661. doi: 10.1126/sciadv.aap9661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans NT, Lamberti GA. Freshwater fisheries assessment using environmental DNA: a primer on the method, its potential, and shortcomings as a conservation tool. Fish. Res. 2018;197:60–66. doi: 10.1016/j.fishres.2017.09.013. [DOI] [Google Scholar]
- 5.Qu C, Stewart KA. Evaluating monitoring options for conservation: comparing traditional and environmental DNA tools for a critically endangered mammal. Sci. Nat. 2019;106:9. doi: 10.1007/s00114-019-1605-1. [DOI] [PubMed] [Google Scholar]
- 6.Roussel JM, Paillisson JM, Treguier A, Petit E. The downside of eDNA as a survey tool in water bodies. J. Appl. Ecol. 2015;52:823–826. doi: 10.1111/1365-2664.12428. [DOI] [Google Scholar]
- 7.Strand DA, et al. Monitoring a Norwegian freshwater crayfish tragedy: eDNA snapshots of invasion, infection and extinction. J. Appl. Ecol. 2019;56:1661–1673. doi: 10.1111/1365-2664.13404. [DOI] [Google Scholar]
- 8.Farrell JA, et al. Environmental DNA monitoring of oncogenic viral shedding and genomic profiling of sea turtle fibropapillomatosis reveals unusual viral dynamics. Commun. Biol. 2021;4:565. doi: 10.1038/s42003-021-02085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowney F, et al. Respiratory health outcomes associated with different grass taxa in the UK. Environ. Epidemiol. 2019;3:342. doi: 10.1097/01.EE9.0000609768.07517.40. [DOI] [Google Scholar]
- 10.Johnson MD, Cox RD, Barnes MA. The detection of a non-anemophilous plant species using airborne eDNA. PLoS ONE. 2019;14:e0225262. doi: 10.1371/journal.pone.0225262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gable L, Ram N, Ram JL. Legal and ethical implications of wastewater monitoring of SARS-CoV-2 for COVID-19 surveillance. J. Law Biosci. 2020;7:lsaa039. doi: 10.1093/jlb/lsaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs D, et al. Wastewater monitoring raises privacy and ethical considerations. IEEE Trans. Technol. Soc. 2021;2:116–121. doi: 10.1109/TTS.2021.3073886. [DOI] [Google Scholar]
- 13.Mtetwa HN, Amoah ID, Kumari S, Bux F, Reddy P. Molecular surveillance of tuberculosis-causing mycobacteria in wastewater. Heliyon. 2022;8:e08910. doi: 10.1016/j.heliyon.2022.e08910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson B. What poo tells us: wastewater surveillance comes of age amid COVID, monkeypox, and polio. Br. Med. J. 2022;378:o1869. doi: 10.1136/bmj.o1869. [DOI] [PubMed] [Google Scholar]
- 15.de Jonge E, et al. The detection of monkeypox virus DNA in wastewater samples in the Netherlands. Sci. Total Environ. 2022;852:158265. doi: 10.1016/j.scitotenv.2022.158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomsen PF, Willerslev E. Environmental DNA—an emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 2015;183:4–18. doi: 10.1016/j.biocon.2014.11.019. [DOI] [Google Scholar]
- 17.Farrell JA, et al. Detection and population genomics of sea turtle species via non-invasive environmental DNA analysis of nesting beach sand tracks and oceanic water. Mol. Ecol. Resour. 2022;22:2471–2493. doi: 10.1111/1755-0998.13617. [DOI] [PubMed] [Google Scholar]
- 18.Venter JC, et al. Environmental genome shotgun sequencing of the Sargasso Sea. Science. 2004;304:66–74. doi: 10.1126/science.1093857. [DOI] [PubMed] [Google Scholar]
- 19.Iliana B, et al. Performance of amplicon and shotgun sequencing for accurate biomass estimation in invertebrate community samples. Mol. Ecol. Resour. 2018;18:1020–1034. doi: 10.1111/1755-0998.12888. [DOI] [PubMed] [Google Scholar]
- 20.O’Neill O. Informed consent and genetic information. Stud. Hist. Phil. Sci. C. 2001;32:689–704. [Google Scholar]
- 21.Chadwick R, Berg K. Solidarity and equity: new ethical frameworks for genetic databases. Nat. Rev. Genet. 2001;2:318–321. doi: 10.1038/35066094. [DOI] [PubMed] [Google Scholar]
- 22.Shickle D. The consent problem within DNA biobanks. Stud. Hist. Phil. Sci. C. 2006;37:503–519. doi: 10.1016/j.shpsc.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Roche PA, Annas GJ. Protecting genetic privacy. Nat. Rev. Genet. 2001;2:392–396. doi: 10.1038/35072029. [DOI] [PubMed] [Google Scholar]
- 24.Duffy DJ. Problems, challenges and promises: perspectives on precision medicine. Brief. Bioinform. 2016;17:494–504. doi: 10.1093/bib/bbv060. [DOI] [PubMed] [Google Scholar]
- 25.Hughes JF, et al. Chimpanzee and human Y chromosomes are remarkably divergent in structure and gene content. Nature. 2010;463:536–539. doi: 10.1038/nature08700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins RL, et al. A structural variation reference for medical and population genetics. Nature. 2020;581:444–451. doi: 10.1038/s41586-020-2287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonney S, et al. Predicting the pathogenicity of novel variants in mitochondrial tRNA with MitoTIP. PLoS Comput. Biol. 2017;13:e1005867. doi: 10.1371/journal.pcbi.1005867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick EM, et al. Specifications of the ACMG/AMP standards and guidelines for mitochondrial DNA variant interpretation. Hum. Mutat. 2020;41:2028–2057. doi: 10.1002/humu.24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urban L, et al. Freshwater monitoring by nanopore sequencing. eLife. 2021;10:e61504. doi: 10.7554/eLife.61504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broman E, Bonaglia S, Norkko A, Creer S, Nascimento FJA. High-throughput shotgun sequencing of eRNA reveals taxonomic and derived functional shifts across a benthic productivity gradient. Mol. Ecol. 2021;30:3023–3039. doi: 10.1111/mec.15561. [DOI] [PubMed] [Google Scholar]
- 31.Sharma K, et al. Conservation and people: towards an ethical code of conduct for the use of camera traps in wildlife research. Ecol. Solut. Evid. 2020;1:e12033. doi: 10.1002/2688-8319.12033. [DOI] [Google Scholar]
- 32.Brittain S, et al. Ethical considerations when conservation research involves people. Conserv. Biol. 2020;34:925–933. doi: 10.1111/cobi.13464. [DOI] [PubMed] [Google Scholar]
- 33.Butler D, Meek P. Camera trapping and invasions of privacy: an Australian legal perspective. Torts Law J. 2013;20:234–264. [Google Scholar]
- 34.Cretois, B., Rosten, C. & Sethi, S. S. Automated speech detection in eco-acoustic data enables privacy protection and human disturbance quantification. Preprint at bioRxiv10.1101/2022.02.08.479660 (2022).
- 35.Meystre SM, Friedlin FJ, South BR, Shen S, Samore MH. Automatic de-identification of textual documents in the electronic health record: a review of recent research. BMC Med. Res. Methodol. 2010;10:70. doi: 10.1186/1471-2288-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynggaard C, et al. Airborne environmental DNA for terrestrial vertebrate community monitoring. Curr. Biol. 2022;32:701–707.e5. doi: 10.1016/j.cub.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chrisman B, et al. The human ‘contaminome’: bacterial, viral, and computational contamination in whole genome sequences from 1,000 families. Sci. Rep. 2022;12:9863. doi: 10.1038/s41598-022-13269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longo MS, O’Neill MJ, O’Neill RJ. Abundant human DNA contamination identified in non-primate genome databases. PLoS ONE. 2011;6:e16410. doi: 10.1371/journal.pone.0016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andres KJ, Sethi SA, Lodge DM, Andrés J. Nuclear eDNA estimates population allele frequencies and abundance in experimental mesocosms and field samples. Mol. Ecol. 2021;30:685–697. doi: 10.1111/mec.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nichols RV, Spong G. An eDNA-based SNP assay for ungulate species and sex identification. Diversity. 2017;9:33. doi: 10.3390/d9030033. [DOI] [Google Scholar]
- 41.Sigsgaard EE, et al. Population characteristics of a large whale shark aggregation inferred from seawater environmental DNA. Nat. Ecol. Evol. 2016;1:0004. doi: 10.1038/s41559-016-0004. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg CS, Strickler KM, Fremier AK. Degradation and dispersion limit environmental DNA detection of rare amphibians in wetlands: increasing efficacy of sampling designs. Sci. Total Environ. 2018;633:695–703. doi: 10.1016/j.scitotenv.2018.02.295. [DOI] [PubMed] [Google Scholar]
- 43.Deiner K, Altermatt F. Transport distance of invertebrate environmental DNA in a natural river. PLoS ONE. 2014;9:e88786. doi: 10.1371/journal.pone.0088786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dell’Anno A, Corinaldesi C. Degradation and turnover of extracellular DNA in marine sediments: ecological and methodological considerations. Appl. Environ. Microbiol. 2004;70:4384–4386. doi: 10.1128/AEM.70.7.4384-4386.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes MA, et al. Environmental conditions influence eDNA persistence in aquatic systems. Environ. Sci. Technol. 2014;48:1819–1827. doi: 10.1021/es404734p. [DOI] [PubMed] [Google Scholar]
- 46.Keskin E. Detection of invasive freshwater fish species using environmental DNA survey. Biochem. Syst. Ecol. 2014;56:68–74. doi: 10.1016/j.bse.2014.05.003. [DOI] [Google Scholar]
- 47.Urban, L. et al. Genomic monitoring of the critically endangered Kākāpō by real-time targeted nanopore sequencing of environmental DNA. Curr. Biol. Dec 2021. 10.2139/ssrn.3977260
- 48.Payne A, et al. Readfish enables targeted nanopore sequencing of gigabase-sized genomes. Nat. Biotechnol. 2021;39:442–450. doi: 10.1038/s41587-020-00746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kovaka S, Fan Y, Ni B, Timp W, Schatz MC. Targeted nanopore sequencing by real-time mapping of raw electrical signal with UNCALLED. Nat. Biotechnol. 2021;39:431–441. doi: 10.1038/s41587-020-0731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weilguny, L. et al. Dynamic, adaptive sampling during nanopore sequencing using Bayesian experimental design. Nat. Biotechnol.10.1038/s41587-022-01580-z (2023). [DOI] [PMC free article] [PubMed]
- 51.Urban, L. et al. Non-invasive real-time genomic monitoring of the critically endangered kākāpō. Preprint at bioRxiv10.1101/2022.11.14.516431 (2022). [DOI] [PMC free article] [PubMed]
- 52.Ram N, Gable L, Ram JL. The future of wastewater monitoring for the public health. Univ. Richmond Law Rev. 2021;56:911. [Google Scholar]
- 53.Randazzo W, Cuevas-Ferrando E, Sanjuán R, Domingo-Calap P, Sánchez G. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. Int. J. Hyg. Environ. Health. 2020;230:113621. doi: 10.1016/j.ijheh.2020.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crits-Christoph A, et al. Genome sequencing of sewage detects regionally prevalent SARS-CoV-2 variants. mBio. 2021;12:e02703–e02720. doi: 10.1128/mBio.02703-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gambhir SS, Ge TJ, Vermesh O, Spitler R, Gold GE. Continuous health monitoring: an opportunity for precision health. Sci. Transl. Med. 2021;13:eabe5383. doi: 10.1126/scitranslmed.abe5383. [DOI] [PubMed] [Google Scholar]
- 56.Dugal L, et al. Individual haplotyping of whale sharks from seawater environmental DNA. Mol. Ecol. Resour. 2022;22:56–65. doi: 10.1111/1755-0998.13451. [DOI] [PubMed] [Google Scholar]
- 57.Fatumo S, et al. A roadmap to increase diversity in genomic studies. Nat. Med. 2022;28:243–250. doi: 10.1038/s41591-021-01672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wasser SK, et al. Elephant genotypes reveal the size and connectivity of transnational ivory traffickers. Nat. Hum. Behav. 2022;6:371–382. doi: 10.1038/s41562-021-01267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Domingues RR, Bunholi IV, Pinhal D, Antunes A, Mendonça FF. From molecule to conservation: DNA-based methods to overcome frontiers in the shark and ray fin trade. Conserv. Genet. Resour. 2021;13:231–247. doi: 10.1007/s12686-021-01194-8. [DOI] [Google Scholar]
- 60.Clare EL, et al. eDNAir: proof of concept that animal DNA can be collected from air sampling. PeerJ. 2021;9:e11030. doi: 10.7717/peerj.11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fantinato C, Gill P, Fonneløp AE. Detection of human DNA in the air. Forensic Sci. Int. Genet. Suppl. Ser. 2022;8:282–284. doi: 10.1016/j.fsigss.2022.10.063. [DOI] [Google Scholar]
- 62.Mercer C, Taylor D, Henry J, Linacre A. DNA accumulation and transfer within an operational forensic exhibit storeroom. Forensic Sci. Int. Genet. 2023;62:102799. doi: 10.1016/j.fsigen.2022.102799. [DOI] [PubMed] [Google Scholar]
- 63.Cortez AD, Bolnick DA, Nicholas G, Bardill J, Colwell C. An ethical crisis in ancient DNA research: insights from the Chaco Canyon controversy as a case study. J. Soc. Archaeol. 2021;21:157–178. doi: 10.1177/1469605321991600. [DOI] [Google Scholar]
- 64.Bardill J, et al. Advancing the ethics of paleogenomics. Science. 2018;360:384–385. doi: 10.1126/science.aaq1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gibbon VE. African ancient-DNA research requires robust ethics and permission protocols. Nat. Rev. Genet. 2020;21:645–647. doi: 10.1038/s41576-020-00285-w. [DOI] [PubMed] [Google Scholar]
- 66.Kaestle FA, Horsburgh KA. Ancient DNA in anthropology: methods, applications, and ethics. Am. J. Phys. Anthropol. 2002;119:92–130. doi: 10.1002/ajpa.10179. [DOI] [PubMed] [Google Scholar]
- 67.Dass MA, et al. Assessing the use of environmental DNA (eDNA) as a tool in the detection of human DNA in water. J. Forensic Sci. 2022;67:2299–2307. doi: 10.1111/1556-4029.15124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pipek OA, et al. Worldwide human mitochondrial haplogroup distribution from urban sewage. Sci. Rep. 2019;9:11624. doi: 10.1038/s41598-019-48093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.M’charek A, Toom V, Jong L. The trouble with race in forensic identification. Sci. Technol. Hum. Values. 2020;45:804–828. doi: 10.1177/0162243919899467. [DOI] [Google Scholar]
- 70.Williams R, Wienroth M. Social and ethical aspects of forensic genetics: a critical review. Forensic Sci. Rev. 2017;29:145–169. [PubMed] [Google Scholar]
- 71.Parry B. New spaces of biological commodification: the dynamics of trade in genetic resources and ‘bioinformation’. Interdiscip. Sci. Rev. 2006;31:19–31. doi: 10.1179/030801806X84228. [DOI] [Google Scholar]
- 72.Fox K. The illusion of inclusion—the ‘All of Us’ research program and Indigenous Peoples’ DNA. N. Engl. J. Med. 2020;383:411–413. doi: 10.1056/NEJMp1915987. [DOI] [PubMed] [Google Scholar]
- 73.Juengst ET, Settersten RA, Fishman JR, McGowan ML. After the revolution? Ethical and social challenges in ‘personalized genomic medicine’. Pers. Med. 2012;9:429–439. doi: 10.2217/pme.12.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Samuel GN, Farsides B. Genomics England’s implementation of its public engagement strategy: blurred boundaries between engagement for the United Kingdom’s 100,000 Genomes project and the need for public support. Public Underst. Sci. 2018;27:352–364. doi: 10.1177/0963662517747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Samuel GN, Farsides B. Public trust and ‘ethics review’ as a commodity: the case of Genomics England Limited and the UK’s 100,000 Genomes project. Med. Health Care Phil. 2018;21:159–168. doi: 10.1007/s11019-017-9810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McConnell, D. & Hardiman, O. Ireland putting profit before people with genomic medicine strategy: state backing private company that will own genetic data of public patients. Irish Times (3 July 2019).
- 77.Gibney, E. The scant science behind Cambridge Analytica’s controversial marketing techniques. Nature News Explainer10.1038/d41586-018-03880-4 (2018).
- 78.Ledford, H. Google health-data scandal spooks researchers. Nature News10.1038/d41586-019-03574-5 (2019) [DOI] [PubMed]
- 79.Hudson M, et al. Rights, interests and expectations: indigenous perspectives on unrestricted access to genomic data. Nat. Rev. Genet. 2020;21:377–384. doi: 10.1038/s41576-020-0228-x. [DOI] [PubMed] [Google Scholar]
- 80.Garrison NA, et al. Genomic research through an indigenous lens: understanding the expectations. Annu. Rev. Genom. Hum. Genet. 2019;20:495–517. doi: 10.1146/annurev-genom-083118-015434. [DOI] [PubMed] [Google Scholar]
- 81.Handsley-Davis M, Kowal E, Russell L, Weyrich LS. Researchers using environmental DNA must engage ethically with indigenous communities. Nat. Ecol. Evol. 2021;5:146–148. doi: 10.1038/s41559-020-01351-6. [DOI] [PubMed] [Google Scholar]
- 82.Moreau Y. Crack down on genomic surveillance. Nature. 2019;576:36–38. doi: 10.1038/d41586-019-03687-x. [DOI] [PubMed] [Google Scholar]
- 83.Seymour M, et al. Acidity promotes degradation of multi-species environmental DNA in lotic mesocosms. Commun. Biol. 2018;1:4. doi: 10.1038/s42003-017-0005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Afgan E, et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018;46:W537–W544. doi: 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jalili V, et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2020 update. Nucleic Acids Res. 2020;48:W395–W402. doi: 10.1093/nar/gkaa434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Koning W, et al. NanoGalaxy: nanopore long-read sequencing data analysis in Galaxy. GigaScience. 2020;9:giaa105. doi: 10.1093/gigascience/giaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data v.0.73 (Babraham Bioinformatics, Babraham Institute, 2010).
- 88.Wick, R. Porechop. GitHubhttps://github.com/rrwick (2017).
- 89.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nurk S, et al. The complete sequence of a human genome. Science. 2022;376:44–53. doi: 10.1126/science.abj6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bentley BP, et al. Divergent sensory and immune gene evolution in sea turtles with contrasting demographic and life histories. Proc. Natl Acad. Sci. USA. 2023;120:e2201076120. doi: 10.1073/pnas.2201076120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ackermann M, et al. The genome of chelonid herpesvirus 5 harbors atypical genes. PLoS ONE. 2012;7:e46623. doi: 10.1371/journal.pone.0046623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herbst LH, et al. Genomic characterization of two novel reptilian papillomaviruses, Chelonia mydas papillomavirus 1 and Caretta caretta papillomavirus 1. Virology. 2009;383:131–135. doi: 10.1016/j.virol.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 95.Mashkour N, Jones K, Wirth W, Burgess G, Ariel E. The concurrent detection of chelonid alphaherpesvirus 5 and Chelonia mydas papillomavirus 1 in tumoured and non-tumoured green turtles. Animals. 2021;11:697. doi: 10.3390/ani11030697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pertea M, et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li H, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brandon MC, et al. MITOMASTER: a bioinformatics tool for the analysis of mitochondrial DNA sequences. Hum. Mutat. 2009;30:1–6. doi: 10.1002/humu.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weissensteiner H, et al. HaploGrep 2: mitochondrial haplogroup classification in the era of high-throughput sequencing. Nucleic Acids Res. 2016;44:W58–W63. doi: 10.1093/nar/gkw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Oven M, Kayser M. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum. Mutat. 2009;30:E386–E394. doi: 10.1002/humu.20921. [DOI] [PubMed] [Google Scholar]
- 101.R Team RStudio: Integrated Development for R (Posit Software, 2020) http://www.rstudio.com/
- 102.Wickham, H., Chang, W. & Wickham, M. H. ggplot2: Create elegant data visualisations using the grammar of graphics. R package version 2 (2016).
- 103.Schneider VA, et al. Evaluation of GRCh38 and de novo haploid genome assemblies demonstrates the enduring quality of the reference assembly. Genome Res. 2017;27:849–864. doi: 10.1101/gr.213611.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sedlazeck FJ, et al. Accurate detection of complex structural variations using single-molecule sequencing. Nat. Methods. 2018;15:461–468. doi: 10.1038/s41592-018-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smolka, M. et al. Comprehensive structural variant detection: from mosaic to population-level. Preprint at bioRxiv10.1101/2022.04.04.487055 (2022).
- 106.Kim D, Song L, Breitwieser FP, Salzberg SL. Centrifuge: rapid and sensitive classification of metagenomic sequences. Genome Res. 2016;26:1721–1729. doi: 10.1101/gr.210641.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morgulis A, Gertz EM, Schäffer AA, Agarwala R. A fast and symmetric DUST implementation to mask low-complexity DNA sequences. J. Comput. Biol. 2006;13:1028–1040. doi: 10.1089/cmb.2006.13.1028. [DOI] [PubMed] [Google Scholar]
- 108.Juul, S. et al. What’s in my pot? Real-time species identification on the MinION. Preprint at bioRxiv10.1101/030742 (2015).
- 109.Knaus BJ, Grünwald NJ. vcfr: a package to manipulate and visualize variant call format data in R. Mol. Ecol. Resour. 2017;17:44–53. doi: 10.1111/1755-0998.12549. [DOI] [PubMed] [Google Scholar]
- 110.Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 2012;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anand L, Rodriguez Lopez CM. ChromoMap: an R package for interactive visualization of multi-omics data and annotation of chromosomes. BMC Bioinform. 2022;23:33. doi: 10.1186/s12859-021-04556-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Greene CS, et al. Understanding multicellular function and disease with human tissue-specific networks. Nat. Genet. 2015;47:569–576. doi: 10.1038/ng.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Spitzer M, Wildenhain J, Rappsilber J, Tyers M. BoxPlotR: a web tool for generation of box plots. Nat. Methods. 2014;11:121–122. doi: 10.1038/nmeth.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1.
Supplementary Tables 1–7.
Data Availability Statement
All Illumina sequenced samples including raw reads are deposited in the NCBI database (https://www.ncbi.nlm.nih.gov/) under BioProject ID PRJNA449022. All Oxford Nanopore sequenced samples including raw reads are deposited in the NCBI database (https://www.ncbi.nlm.nih.gov/) under BioProject ID PRJNA874696.