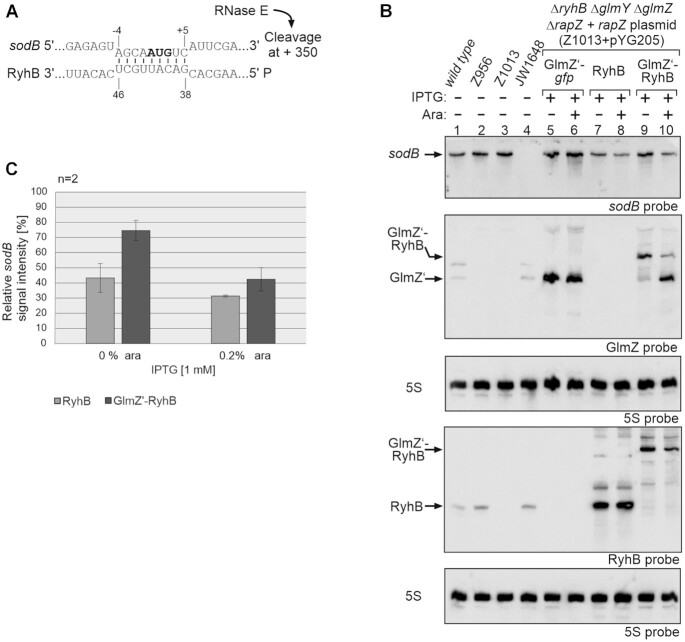
Figure 4.
Analysis of sodB mRNA repression caused by 5′PPP- and 5′P-RyhB. (A) RNA duplex formed by RyhB with the sodB start region (58). RNase E cleavage of sodB mRNA downstream in the coding region is indicated. (B) Northern blot experiment addressing sodB mRNA steady state levels in presence of the GlmZ’-gfp control fusion, 5′PPP-RyhB and 5′P-RyhB, respectively. Strain Z1013 (ΔryhB ΔglmY ΔglmZ ΔrapZ) containing plasmid pYG205 (rapZ), carried additionally either plasmid pYG215 (glmZ’-gfp, lane 5, 6), pYG275 (ryhB, lanes 7, 8) or plasmid pYG274 (glmZ’-ryhB, lanes 9, 10). IPTG (1 mM) was added to induce expression of corresponding RNA constructs, while expression of rapZ from pYG205 was induced with 0.2% arabinose where indicated (+). The empty ancestor strains S4197 (wild-type), Z956 (ΔglmY ΔglmZ ΔrapZ), Z1013 and JW1648 (ΔsodB) were included as additional controls (lanes 1–4). Total RNAs were isolated and 1.5 μg each were separated on two denaturing 8% PAA gels. After blotting, the membranes were either hybridized with probes detecting sodB and GlmZ (first and second panel from top), or probed for RyhB (fourth panel from top). Blots were re-probed for 5S rRNA to obtain loading controls. (C) Quantification of Northern blots. Bar graphs presenting the average normalized signal intensities and SD of two independent experiments. sodB mRNA signal intensities, in presence of RyhB (light grey) and GlmZ’-RyhB (dark grey), were compared to sodB signal intensity obtained in presence of GlmZ’-gfp (lane 5 and 6; =100%) and normalized to the corresponding 5S rRNA loading controls.