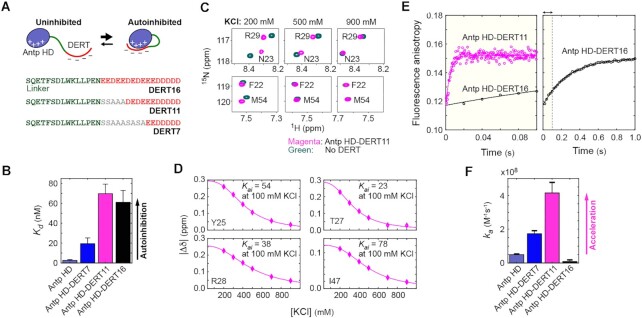
Figure 3.
Artificial autoinhibitory systems using D/E repeat tail (DERT). (A) Protein constructs of the Antp homeodomain with a DERT (red) attached. The sequence shown in green is a linker adopted from p53 residues 15–29. (B) The dissociation constants (Kd) for the complexes of the protein constructs with 15-bp DNA containing the Antp recognition sequence. (C) Overlaid heteronuclear 1H–15N correlation spectra recorded for Antp HD-DERT11 and the control protein with no DERT at various concentrations of KCl. Due to intra-molecular electrostatic interactions between HD and DERT, the NMR chemical shifts of the two constructs are significantly different at lower ionic strengths. (D) Chemical shift differences between Antp HD-DERT11 and the control protein with no DERT. The autoinhibition equilibrium constant Kai at 100 mM KCl was determined through the fitting to the KCl concentration dependence of chemical shifts for each residue, as previously described (13). The solid curves represent the best-fit curve. Variation in the Kai constant among different residues may reflect the dynamic nature of DERT in the autoinhibited state (13). (E) Stopped-flow fluorescence anisotropy data measured upon mixing 200 nM protein with a solution of 10 nM 33-bp FAM-labeled DNA (which contains an Antp recognition sequence) and 4 μM 15-bp decoy DNA at 100 mM KCl. Because Antp HD-DERT11 exhibited very fast kinetics, the time interval between anisotropy measurements was set to a smaller value for this protein. The larger noise is due to the shorter time interval. (F) Apparent association rate constant ka determined using the protein concentration dependence data from the stopped-flow fluorescence kinetics experiments.