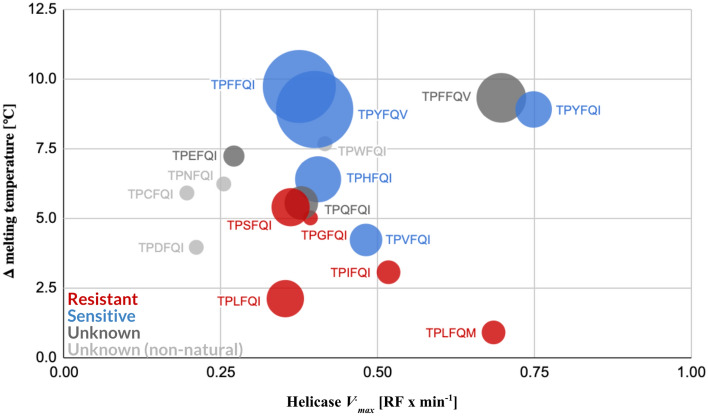
Figure 5.
Shifts in thermal denaturation temperature of different eIF4A:RNA:silvestrol complexes are associated with eIF4A sensitivity to rocaglates. While relative eIF4A helicase activities of eIF4A mutant proteins expressing different rocaglate-binding aa patterns fall within a narrow range of Vmax values and do not correlate with rocaglate sensitivity, comparative thermal shift analysis of the mutant proteins showed a clear association between sensitivity to rocaglates and higher thermal denaturation differentials between the eIF4A:RNA and eIF4A:RNA:silvestrol complexes. The increased stability of the rocaglate-sensitive mutants is determined by π–π-stacking interactions elicited by the corresponding aa residues at position 163. Data points represent mean values of three technical replicates. Standard errors for the helicase activities are indicated in Fig. 4A and standard errors for the Δ melting temperature are listed in Table S5. The size of the circles denotes prevalence of the aa pattern among the eIF4As included in our survey.