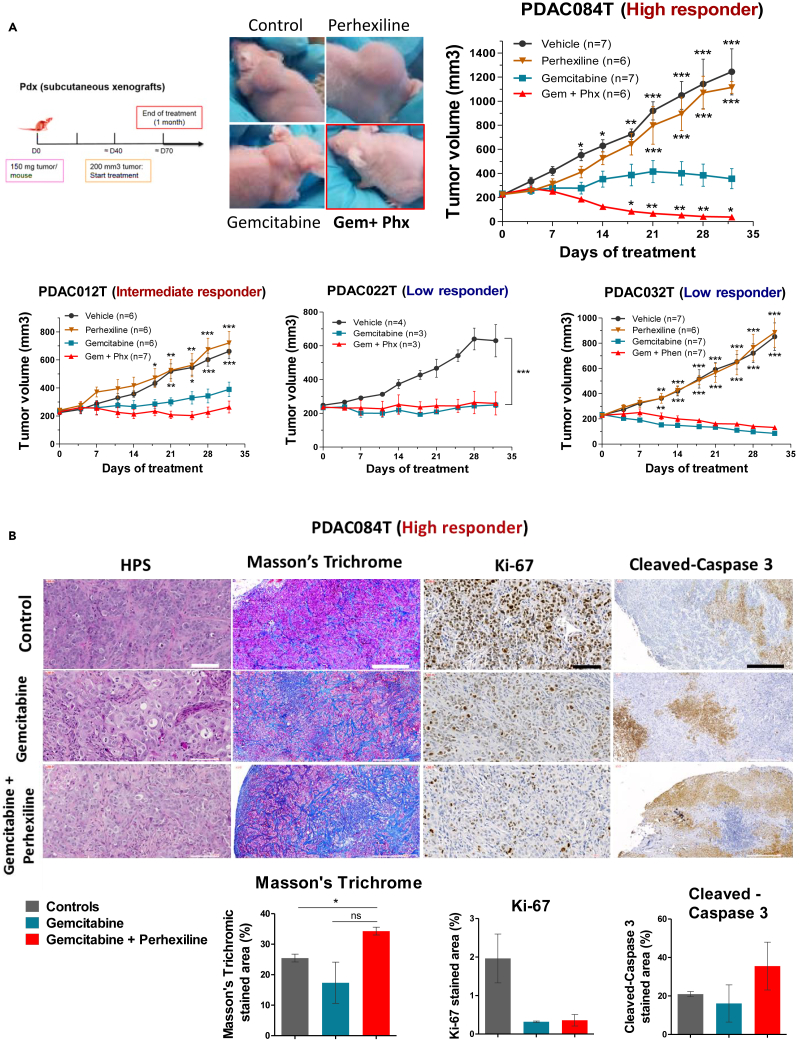
Figure 4.
Perhexiline in combination with gemcitabine induces complete pancreatic cancer regression in vivo in the high responder PDAC084T xenograft
(A) Left. Schematic of experimental protocol. Pieces of tumor from PDX were implanted in the subcutaneous space of recipient female Swiss nude mice. When tumors reached 200 mm3 volume, mice were assigned to treatment groups and treated for one month. Right and below. Tumor volume in four different PDAC PDX (PDAC084T, PDAC012T, PDAC022T, and PDAC032T) treated during one month with gemcitabine (120 mg/kg IP twice a week), perhexiline (5 mg/kg IP every other day), gemcitabine plus perhexiline (with the same indications), and vehicle. PDAC084T (high responder). Photos of representative tumor-bearing mice (left) and tumor volume during one month treatment (right). Perhexiline not only enhances the antitumoral effect of gemcitabine but also results in complete tumor regression. PDAC012T (intermediate responder). The combination treatment shows a better efficiency than gemcitabine alone. PDAC022T and PDAC032T (low responders). No effect was seen upon combination treatment compared to gemcitabine alone. Data are presented as mean ± SEM. ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05 from two-way ANOVA test, compared to gemcitabine treatment alone.
(B) Representative microscopic images from PDAC084T tumor sections obtained in the middle-point of treatment with gemcitabine or gemcitabine plus perhexiline. Tissue sections were stained with hematoxylin phloxine saffron (HPS) and Masson’s Trichrome for histologic examination and fibrosis detection, respectively. Immunohistochemistry was performed staining Ki-67 and cleaved caspase-3 for proliferation and apoptosis detection, respectively. Scale bars: 100 μM for HPS and Ki-67, and 500 μM for Masson’s Trichrome and cleaved caspase-3. Quantification is shown below, and data are the mean ± SEM of the percentage of stained area of tumors from at least two mice per group. p values calculated from t test; ∗p < 0.05.