Abstract
Objective: The aim of this study was to evaluate the residual volume of liver reserve function in liver cancer patients using three-dimensional reconstruction technique (3D technology) and the indocyanine green (ICG) excretion test. Methods: A retrospective analysis was conducted, and data were collected from 90 liver cancer patients in Ganzhou People’s Hospital between January 2017 and December 2021. The control group underwent preoperative resectability evaluation based on traditional two-dimensional images, whereas the experimental group underwent digital three-dimensional reconstruction technique combined with indocyanine green (ICG) excretion test. The intraoperative bleeding volume, accuracy of preoperative surgical planning, operation time, postoperative complication rate, and perioperative mortality were compared between the two groups. Results: The assessment of resected liver volume (resectability) in the experimental group was larger than in the control group (P=0.003). Moreover, the accuracy rate of preoperative surgical planning in the experimental group was higher than in the control group (P=0.014). The intraoperative estimated blood loss favored the experimental group by a mean of 355 ml (P=0.02). Operative time and hospital stay favored the experimental group by a mean time of 204 min (P=0.03). The positive rate of liver resection margin and recurrence rate in the experimental group were lower than in the control group (P=0.021, P=0.004). Moreover, the two groups differed after intervention in terms of AST (P=0.001), ALT (P=0.0001), TBIL (P=0.001), and ALB (P=0.026). Conclusion: The combination of three-dimensional reconstruction technique and indocyanine green (ICG) excretion test provides accurate visualization of hepatic anatomy and improves the precision of liver resection surgery, which is of great guiding value. This can optimize the preoperative evaluation and surgical planning for liver resection, shorten the operation time, and reduce intraoperative bleeding volume.
Keywords: Digital three-dimensional reconstruction technique, indocyanine green (ICG) excretion test, sorafenib, precision hepatectomy, primary liver cancer
Introduction
Primary hepatocellular carcinoma (HCC) is a highly prevalent cancer type with a poor prognosis, often leading to death within weeks or months of diagnosis. The incidence of liver cancer is particularly high in China, with an estimated 1 million new cases added worldwide each year [1,2]. Currently, partial hepatectomy is considered the most effective way to cure liver cancer and offers patients the best chance for a complete recovery [3]. In China, over 75% of liver cancer cases occur in conjunction with cirrhosis [4]. However, due to varying preoperative evaluation protocols and surgical capabilities across different hospitals, many patients who are suitable for partial hepatectomy instead receive palliative treatments like TACE [5,6]. Therefore, it is essential to ensure adequate and precise preoperative evaluation and hepatectomy techniques to improve the radical resection rate and overall prognosis for liver cancer patients.
Precision liver surgery has revolutionized the field of hepatic surgery. With a focus on complete removal of target lesions, precision liver surgery ensures the preservation of the remaining liver anatomic structure and optimizes the functional liver volume while minimizing surgical bleeding and systemic trauma invasion, ultimately leading to the best rehabilitation outcomes [7,8]. However, the key to achieving accurate hepatectomy lies in the proper assessment of liver reserve function and residual liver volume, as well as a thorough understanding of intrahepatic and tumor anatomic structures [9]. The ICG R15 (indocyanine green retention rate at 15 minutes) is a quantitative indicator that has been widely accepted for assessing liver reserve function, and it has been shown to be a prognostic factor for liver cirrhosis patients undergoing hepatectomy [10]. Many researchers have proposed safety limit evaluation systems for hepatectomy based on the ICG R15 [11,12].
The digital three-dimensional (3D) reconstruction technique provides a clear and realistic display of the size, location, and shape of liver tumors, as well as their spatial relationship with the intrahepatic vascular and biliary systems [13]. It can also perform individualized liver segmentation based on the 3D images to reveal the course of the Glisson system and the precise location of the tumor in the liver segment relative to the blood vessels [14,15]. The visualization of intrahepatic and tumor anatomic structures using digital 3D technology greatly enhances the accuracy of surgical planning for hepatectomy [16]. For instance, the clear display of the S4 hepatic vein provides a reliable basis for performing right hemihepatectomy or right third hepatectomy [17]. The imaging of the right posterior inferior vein can prevent inadvertent resection of S7 and 8 tumors by enlarging the right half of the liver, allowing for combined resection instead to achieve radical resection [17]. Similarly, the display of the portal vein helps surgeons avoid injuring or cutting off the right anterior branch during left hemihepatectomy, which could otherwise lead to postoperative liver failure and complications [18].
The digital three-dimensional (3D) reconstruction technique uses computer image processing to transform 2D sectional image sequences from ultrasound, computed tomography, and magnetic resonance imaging into 3D images that enable deep mining, prediction, and analysis of massive image data files, leading to highly accurate diagnoses [19]. Compared to traditional 2D imaging techniques, digital 3D reconstruction provides a more intuitive, clear, and multi-angled view that includes an intrahepatic vascular display system, allowing for precise identification of lesion locations, surgical margins, and liver volume estimation. Furthermore, this technology enables surgical simulations and is beneficial in preoperative evaluations, surgical planning, and guidance for precise liver resection strategies. For instance, Igami et al. employed 3D printed transparent liver models to identify small liver cancers that could not be detected by intraoperative ultrasound, demonstrating promising results for the surgical removal of these tiny tumors [20]. While the digital 3D reconstruction technique has shown excellent results in precision liver surgery, it is still in the developmental stages, and its efficacy and safety need to be further investigated.
The objective of this study was to assess residual liver volume and liver reserve function in patients with liver cancer, using both 3D technology and ICG R15 technology. The visualization provided by 3D technology guides hepatectomy surgical planning, leading to increased rates of surgical resection and radical resection, as well as improved accuracy and safety during surgery. This approach reduces surgical trauma and postoperative complications in liver cancer patients and decreases tumor recurrence rates following surgery. Ultimately, the findings of this study contribute towards developing a more effective and streamlined hepatectomy procedure for patients with liver cancer.
Data and methods
Study design and patients
This study was approved by the ethics committee of Ganzhou People’s Hospital and adopted a retrospective cohort study design. Through the electronic medical record system, 212 patients who underwent precision hepatectomy in primary liver cancer in Ganzhou People’s Hospital from January 2017 to December 2021 were selected according to the following inclusion criteria: 1) Patients with liver cancer defined as detection of typical liver cancer imaging features or blood indicators based on the clinical criteria for probable liver cancer as described by the National Institute on cancer’s Association [21]. 2) Patients aged ≥18 years. 3) Patients with liver function Child Pugh grade A or B, improved to grade A after short-term liver protection treatment. 4) Patients without extensive extrahepatic metastasis. 5) Patients with less than 5 tumor lesions, confined to either 2-3 adjacent liver segments or half of the liver. 6) Patients undergo the ICG excretion test in combination with either digital three-dimensional reconstruction technique or traditional two-dimensional images, as a form of preoperative evaluation method. 7) Patients with complete basic information and laboratory examination data.
We excluded 1) patients who are in poor condition or have severe heart, lung, kidney, or other important organ diseases or may not be able to undergo surgery due to their health status, 2) patients with liver function categorized as Child-Pugh Grade C or B who may not be able to improve to Grade A even after undergoing short-term liver protection treatment, 3) patients with extensive intrahepatic or extrahepatic metastasis, 4) patients with tumors that have invaded the first and second hepatic hilum, 5) patients with recurrent liver cancer, or 6) patients with incomplete clinical data.
The dataset consisting of 90 patients was divided into two groups, experimental (n=50) and control (n=40), based on the preoperative evaluation method. The experimental group was subjected to the digital three-dimensional reconstruction technique combined with indocyanine green (ICG) excretion test. In this process, the three-dimensional models of patients were observed from multiple angles by the researcher, who also remembered the preoperative surgical plan. Various values such as total liver volume, segmental volumes, liver parenchymal volume, tumor volume, estimated volume of resected liver, reserved liver volume, and resection rate of liver parenchyma were calculated (as shown in Figure 1). The operation mode was determined using the ICG 15 test. On the other hand, the control group received preoperative resectability evaluation based on traditional two-dimensional images combined with indocyanine green (ICG) excretion test. The surgeon made a comprehensive assessment of various factors such as the patient’s general condition, Child-Pugh grade, portal hypertension, transaminase level, hepatitis B DNA level, CT or MRI three-phase enhanced scanning. This evaluation was jointly completed by two chief physicians of hepatobiliary surgery and one senior associate chief physician of imaging specialty. Finally, the hepatectomy plan was drawn up before the operation. A flow diagram detailing the selection of patients is shown in Figure 2.
Figure 1.
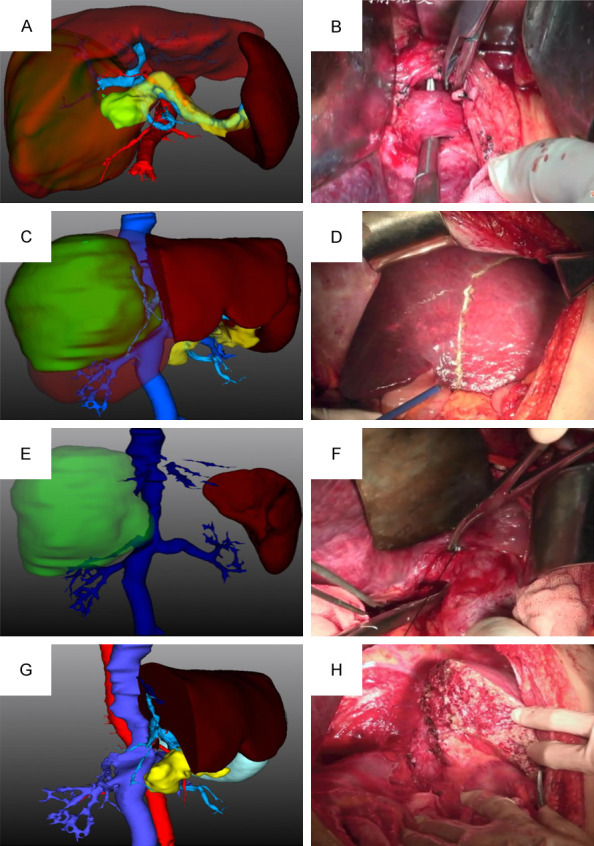
The digital three-dimensional reconstruction technique. A, B: Digital three-dimensional visualization showed the spatial relationship between the right portal vein branch and the right hepatic artery, as well as intrahepatic migration, which was consistent with that during the operation. C, D: Digital three-dimensional visualization showed intrahepatic duct structure at the level of liver transection. E, F: Digital three-dimensional visualization that showed right posterior inferior hepatic vein was consistent with that during operation. G, H: Digital three-dimensional visualization that showed guiding surgical planning was consistent with the actual surgical findings.
Figure 2.

Flow chart showing recruitment.
Data collection
Preoperative data of eligible patients, including gender, age, BMI, comorbid conditions (coronary heart disease, hypertension, diabetes, coronary heart disease and smoking history) were collected from the patient records. Moreover, we recorded the 3D reconstruction of thin slice CT data and indocyanine green (ICG) excretion test.
Intraoperative data obtained from anesthesia and medical records included intraoperative estimated blood loss and operation time.
The clinical data of one day after surgery, which comprised liver function, positive liver resection margin, recrudescence, and hospital stay, and mortality within 30 days after surgery were also extracted.
Outcome measures
The primary indication included: resectability and the accuracy of preoperative surgical planning, whichwere evaluated by 2D imaging and 3D combined with ICG 15.
The secondary indication included: Intraoperative bleeding volume; Operation time; Postoperative complication rate; perioperative mortality; R0 resection rate; hospital stay; survival rate; general characteristics of the participants, ALT, AST, TBIL, and ALB of liver function after operation. All indicators were tested and evaluated before and one week after operation.
Statistical analysis
SPSS13.0 statistical software was used for data statistics. The ratio of rates between the two groups was determined by χ2 test. The measured data between groups were analyzed by ANOVA. The correlation between the two was expressed by contingency coefficient C. The closer C is to 1, the stronger the correlation is. P<0.05 was considered significant.
Results
Clinical data of the participants
Table 1 shows characteristics of the participants. Among those cases, we excluded: 27 cases aged <18 years old; 12 cases with extensive extrahepatic metastasis; 9 cases with tumor invaded the first and second hepatic hilum before the operation; 7 cases with recurrent liver cancer; 5 cases who undergo preoperative evaluation but did not undergo surgery due to that the evaluation showed high risk; and 62 cases with incomplete clinical data. Finally, a total of 90 cases were included, involving 50 patients in the experimental group with a mean age (45.1±12.3) years old, and 40 in the control group with a mean age of (42.5±11.47) years old. There was no significant difference between the two groups in terms of gender, smoking, cerebral infarction, hypertension, diabetes, coronary heart disease, hemoglobin, BMI, platelet count, albumin, TB, DB, ALT, AST, creatinine, PT orINR level.
Table 1.
Comparison of clinical data between the two groups
| Experimental group (n=50) | Control group (n=40) | t/χ2 | P | |
|---|---|---|---|---|
| Age (years) | 45.1±12.3 | 42.5±11.47 | 2.25 | 0.65 |
| Sex | 1.156 | 0.64 | ||
| Male (n%) | 29 (58.0%) | 24 (60.0%) | ||
| Female (n%) | 21 (42.0%) | 16 (40%) | ||
| BMI | 19.4±1.66 | 19.1±1.76 | 5.74 | 0.21 |
| Hemoglobin (g/L) | 120±145 | 123±148 | 5.64 | 0.22 |
| Platelet count (×10/L) | 110±152 | 117±203 | 7.43 | 0.13 |
| Creatinine (μ mol/L) | 51±68 | 52±71 | 5.72 | 0.31 |
| PT (sec) | 13.4±15.2 | 13±14.2 | 2.43 | 0.62 |
| INR | 1.02±1.21 | 0.99±1.11 | 3.12 | 0.609 |
| Smoking | 16 (32.0%) | 16 (40.0%) | 2.71 | 0.35 |
| Cerebral infarction | 7 (14.0%) | 7 (17.5%) | 2.96 | 0.33 |
| Hypertension | 17 (34.0%) | 17 (42.5%) | 1.79 | 0.26 |
| Diabetes | 10 (20.0%) | 11 (27.5%) | 1.29 | 0.19 |
| Coronary heart disease | 4 (8.0%) | 6 (15.0%) | 2.48 | 0.32 |
Note: Significant difference defined as P<0.05.
Comparative perioperative indexes between the two groups
The intraoperative estimated blood loss favored the experimental group by a mean of 355 ml (P=0.02) (Table 2). Operative time and hospital stay favored the experimental group by a mean time of 204 min (P=0.03).
Table 2.
Comparison of perioperative related indexes between two groups
| Experimental group (n=50) | Control group (n=40) | t | P | |
|---|---|---|---|---|
| Intraoperative estimated blood loss (ml) | 355.1±213.14 | 435.4±231.16 | -2.23 | 0.02 |
| Operation time (min) | 204.3±64.13 | 295.6±45.17 | -2.17 | 0.03 |
| Hospital stay | 8.82±1.06 | 11.34±2.98 | 7.985 | 0.01 |
Comparison of postoperative complications
There were significant differences between the two groups in terms of the symptoms of bile leakage of liver wound (P=0.018), postoperative bleeding (P=0.034), hepatic failure (P=0.026), incision infection (P=0.029), pulmonary infection (P=0.031) and rash (P=0.022) (Table 3).
Table 3.
Comparison of postoperative complications between the two groups
| Experimental group (n=50) | Control group (n=40) | χ2 | P | |
|---|---|---|---|---|
| Bile leakage of liver wound | 2 (4.0%) | 3 (7.5%) | 8.98 | 0.018 |
| Postoperative bleeding | 6 (12.0%) | 7 (17.5%) | 7.88 | 0.034 |
| Hepatic failure | 2 (4.0%) | 10 (25.0%) | 8.58 | 0.026 |
| Incision infection | 3 (6.0%) | 5 (12.5%) | 7.45 | 0.029 |
| Pulmonary infection | 9 (18.0%) | 11 (27.5%) | 6.84 | 0.031 |
| Total (occurrence rate) | 22 (44.0%) | 36 (90.0%) | 8.98 | 0.022 |
Comparison of liver function
As shown in the Table 4, there were significant differences between the two groups after intervention in terms of AST (P=0.001), ALT (P=0.0001), TBIL (P=0.001), and ALB (P=0.026) (Table 4). This indicated that liver function was more improved in the experimental group after intervention.
Table 4.
Comparison of liver function between the two groups after intervention
| Time | Experimental group (n=50) | Control group (n=40) | t | P | |
|---|---|---|---|---|---|
| ALT (U/L) | Before intervention | 75.17±11.32 | 73.08±11.28 | 0.029 | 0.977 |
| After intervention | 42.25±2.33 | 61.74±5.62 | 21.007 | 0.0001 | |
| TBIL (μ mol/L) | Before intervention | 19.88±2.63 | 19.78±2.71 | 0.174 | 0.863 |
| After intervention | 22.25±0.65 | 28.77±1.02 | 31.749 | 0.001 | |
| AST (U/L) | Before intervention | 43.21±10.65 | 43.18±10.58 | 0.013 | 0.990 |
| After intervention | 29.32±6.52 | 36.48±7.69 | 4.657 | 0.001 | |
| ALB (g/L) | Before intervention | 38.12±5.32 | 38.07±5.22 | 0.044 | 0.965 |
| After intervention | 35.65±10.02 | 31.32±7.52 | 2.265 | 0.026 | |
| R15 (%) | Before intervention | 7.1±8.1 | 6.9±5.2 | 0.064 | 0.668 |
| After intervention | 16.65±10.1 | 11.32±9.5 | 3.245 | 0.013 | |
| ΔR15 | - | 9.6±8.8 | 4.4±3.9 | 5.784 | 0.001 |
Note: AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; TBIL: total bilirubin; ALB: albumin.
Comparison of positive margin of liver resection, recurrence rate, and mortality
The positive rate of liver resection margin and recurrence rate in the experimental group were lower than in the control group (P=0.021, P=0.004) (Table 5).
Table 5.
Comparison of positive margin of liver resection, recurrence rate, and mortality between the two groups
| Experimental group (n=50) | Control group (n=40) | χ2 | P | |
|---|---|---|---|---|
| Positive liver Resection margin | 0 (0.00%) | 5 (11.63%) | 5.309 | 0.021 |
| Recurrence | 4 (9.30%) | 15 (34.88%) | 8.174 | 0.004 |
| Death | 2 (4.65%) | 6 (13.95%) | 2.205 | 0.138 |
Comparison of accuracy of the two groups
The assessment of resected liver volume (resectability) in the experimental group was larger than in the control group ((255.32±81.2) vs. (201.32±91.5), P=0.003); moreover, the accuracy rate of preoperative surgical planning in the experimental group was higher than control group (P=0.014) (Table 6), indicating the digital three-dimensional reconstruction technique combined with indocyanine green (ICG) excretion test could improve the accuracy of preoperative surgical planning.
Table 6.
Comparison of accuracy between the two groups
| Experimental group (n=50) | Control group (n=40) | χ2 | P | |
|---|---|---|---|---|
| Assessment of resected liver volume | 255.32±81.2 | 201.32±91.5 | 13.345 | 0.003 |
| Remaining (functional) liver volume | 869.6±58.8 | 877.4±78.9 | 2.784 | 0.121 |
| Actual resected liver volume | 246.23±71.3 | 253.32±61.5 | 2.205 | 0.138 |
| Accurate resection rate | 48 (96%) | 27 (67.5%) | 9.194 | 0.014 |
Discussion
Surgery is the primary treatment for primary liver cancer, such as hepatectomy. This involves removing the liver segment, liver lobe, or semi-liver and any other tissues that contain tumors, while retaining enough normal liver tissue to maintain liver function [22]. However, traditional hepatectomy can leave large surgical trauma that affects the patient’s postoperative recovery and liver function, leading to a poor prognosis [23]. In recent years, there has been a shift towards precision and minimally invasive surgery in hepatobiliary surgery. By proposing preoperative accurate evaluation and individualized programs, surgeons can avoid intraoperative injuries and reduce intraoperative bleeding, while providing maximum protection to the liver [24,25]. The digital liver 3D reconstruction system enables doctors to achieve quantitative analysis of liver data, and make precise surgical planning based on analysis results, for surgery to be performed in accordance with the previously proposed plan [26-29]. With digital three-dimensional reconstruction technology, doctors can understand the residual volume of the patient’s liver before surgery, observe the tumor position and intrahepatic vessel displacement from multiple angles, simulate the cutting of tumor tissue, and timely adjust the surgical plan, all of which improves the safety of surgery [30]. Results show that laparoscopic precision hepatectomy based on digital three-dimensional technology significantly reduces intraoperative blood flow when compared to laparoscopic precision hepatectomy alone. Precise preoperative evaluation based on three-dimensional reconstruction technology provides a reference for planning laparoscopic precision hepatectomy, maximizing the removal of lesions, reducing damage to surrounding organs, retaining normal liver tissue to the maximum extent, reducing bleeding, and shortening hospital stay and anal exhaust time.
According to relevant research [31], postoperative recurrence and metastasis of liver cancer patients may be related to the surgical margin. However, there is no consensus on a safe margin for early hepatectomy in patients with primary liver cancer. Improper selection of the margin may lead to recurrence and affect patient prognosis. While radical resection of tumors requires a larger cutting edge for thorough removal of focus tissue, a large cutting edge in primary liver cancer patients can reduce liver volume and damage liver function, leading to decompensation [32]. Thus, accurately grasping the scope of hepatectomy has always been a focus of clinical research [33,34]. Laparoscopic precise hepatectomy implemented in this study, assisted by digital three-dimensional reconstruction technology, helps to fully understand the spatial relationship between vascular system and tumor, achieve more accurate anatomical hepatectomy, reduce the positive rate of liver resection margin, and improve survival rates [35-37]. Results showed that laparoscopic precise hepatectomy based on digital three-dimensional reconstruction technology led to lower rates of positive margins and recurrence when compared to the control group, indicating improved prognosis for patients with primary liver cancer. Moreover, the surgical scheme can be optimized under the guidance of digital three-dimensional reconstruction technology, enabling more accurate and reliable handling of sectional blood vessels and bile duct structures to reduce liver damage. Comparison of liver function indicators further showed that postoperative ALT, TBIL, and AST were lower in the observation group than the control group, while ALB was higher, suggesting that laparoscopic precise hepatectomy with digital three-dimensional reconstruction technology can improve liver function.
ICG uses infrared rays to monitor the concentration of light-absorbing substances in the blood, and indocyanine green is a superior red-light-sensitive dye that accurately measures the concentration of indocyanine green in the blood, reflecting the functional state of liver cells [38]. Factors affecting ICG clearance rate include blood flow velocity, liver cell function, and number. Liver reserve function post-hepatectomy mainly depends on the reserve function of the original liver before surgery and the weakening of liver function due to the operation [39]. Surgery affects residual liver reserve function mainly through two aspects: ischemia-reperfusion injury caused by blocking blood flow to reduce bleeding during surgery and hypoxia injury caused by intraoperative bleeding [40]. Currently, ICG is widely used to evaluate preoperative liver reserve function. In this study, there was no significant difference in preoperative R15 between the two groups, indicating equivalent liver reserve function before surgery. Post-surgery, ΔR15 in the control group was significantly higher than the experimental group (P<0.05), indicating a protective function on liver reserve function in the experimental group.
Undoubtedly, our investigation has certain limitations. First, this study is retrospective, and data sources are limited by the medical record system. As a result, some significant medical record data of patients were not included, including control of concomitant diseases, trends in laboratory test indicators, types and dosages of drugs, course of treatment, and emotional and psychological state of patients at the onset of symptoms. Second, as the research population originates from a single hospital, the study has certain constraints. Therefore, a large-scale multicenter study is necessary for further validation.
In summary, the combination of digital three-dimensional reconstruction technique with the indocyanine green (ICG) excretion test for primary liver cancer offers several advantages. It helps to shorten the operation time and minimize bleeding during the procedure. Furthermore, it enables more precise resection of lesions resulting in a greater improvement in liver function while protecting the residual liver more accurately and safely.
Disclosure of conflict of interest
None.
References
- 1.Grandhi MS, Kim AK, Ronnekleiv-Kelly SM, Kamel IR, Ghasebeh MA, Pawlik TM. Hepatocellular carcinoma: from diagnosis to treatment. Surg Oncol. 2016;25:74–85. doi: 10.1016/j.suronc.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Wallace MC, Preen D, Jeffrey GP, Adams LA. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol. 2015;9:765–779. doi: 10.1586/17474124.2015.1028363. [DOI] [PubMed] [Google Scholar]
- 3.Zhong F, Cheng XS, He K, Sun SB, Zhou J, Chen HM. Treatment outcomes of spontaneous rupture of hepatocellular carcinoma with hemorrhagic shock: a multicenter study. Springerplus. 2016;5:1101. doi: 10.1186/s40064-016-2762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris PS, Hansen RM, Gray ME, Massoud OI, McGuire BM, Shoreibah MG. Hepatocellular carcinoma surveillance: an evidence-based approach. World J Gastroenterol. 2019;25:1550–1559. doi: 10.3748/wjg.v25.i13.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahbari NN, Mehrabi A, Mollberg NM, Müller SA, Koch M, Büchler MW, Weitz J. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253:453–469. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- 6.Sadot E, Lee SY, Sofocleous CT, Solomon SB, Gönen M, Kingham TP, Allen PJ, DeMatteo RP, Jarnagin WR, Hudis CA, D’Angelica MI. Hepatic resection or ablation for isolated breast cancer liver metastasis: a case-control study with comparison to medically treated patients. Ann Surg. 2016;264:147–154. doi: 10.1097/SLA.0000000000001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ni ZK, Lin D, Wang ZQ, Jin HM, Li XW, Li Y, Huang H. Precision liver resection: three-dimensional reconstruction combined with fluorescence laparoscopic imaging. Surg Innov. 2021;28:71–78. doi: 10.1177/1553350620954581. [DOI] [PubMed] [Google Scholar]
- 8.Takamoto T, Makuuchi M. Precision surgery for primary liver cancer. Cancer Biol Med. 2019;16:475–485. doi: 10.20892/j.issn.2095-3941.2019.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsharif M, Roche M, Wilson D, Basak S, Rowe I, Vijayanand D, Feltbower R, Treanor D, Roberts L, Guthrie A, Prasad R, Gilthorpe MS, Attia M, Sourbron S. Hepatectomy risk assessment with functional magnetic resonance imaging (HEPARIM) BMC Cancer. 2021;21:1139. doi: 10.1186/s12885-021-08830-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizumoto M, Oshiro Y, Okumura T, Fukuda K, Fukumitsu N, Abei M, Ishikawa H, Ohnishi K, Numajiri H, Tsuboi K, Sakurai H. Association between pretreatment retention rate of indocyanine green 15 min after administration and life prognosis in patients with HCC treated by proton beam therapy. Radiother Oncol. 2014;113:54–59. doi: 10.1016/j.radonc.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Seyama Y, Kokudo N. Security liver resection of liver function evaluation. Liver Disease Studies. 2009;39:107–116. doi: 10.1111/j.1872-034X.2008.00441.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang XQ, Liu Z, Lv WP, Luo Y, Yang GY, Li CH, Meng XF, Liu Y, Xu KS, Dong JH. Safety validation of decision trees for hepatocellular carcinoma. World J Gastroenterol. 2015;21:9394–9402. doi: 10.3748/wjg.v21.i31.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang CH, Tao HS, Yang J, Fang ZS, Cai W, Liu J, Fan YF. Impact of three-dimensional reconstruction technique in the operation planning of centrally located hepatocellular carcinoma. J Am Coll Surg. 2015;220:28–37. doi: 10.1016/j.jamcollsurg.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Fang C, An J, Bruno A, Cai X, Fan J, Fujimoto J, Golfieri R, Hao X, Jiang H, Jiao LR, Kulkarni AV, Lang H, Lesmana CRA, Li Q, Liu L, Liu Y, Lau W, Lu Q, Man K, Maruyama H, Mosconi C, Örmeci N, Pavlides M, Rezende G, Sohn JH, Treeprasertsuk S, Vilgrain V, Wen H, Wen S, Quan X, Ximenes R, Yang Y, Zhang B, Zhang W, Zhang P, Zhang S, Qi X. Consensus recommendations of three-dimensional visualization for diagnosis and management of liver diseases. Hepatol Int. 2020;14:437–453. doi: 10.1007/s12072-020-10052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang N, Fang C, Fan Y, Yang J, Zeng N, Liu J, Zhu W. Application of liver three-dimensional printing in hepatectomy for complex massive hepatocarcinoma with rare variations of portal vein: preliminary experience. Int J Clin Exp Med. 2015;8:18873–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou XJ, Dong Q, Zhu CZ, Chen X, Wei B, Duan YH, Zhao J, Hao XW, Zhang H, Nie P, Hu B, Xu WJ, Shen RW, Chen ZH, Dong KR, Bai YZ, Shu Q, Luo WJ, Gao F, Xia N, Yu QY. The role and significance of digital reconstruction technique in liver segments based on portal vein structure. Zhonghua Wai Ke Za Zhi. 2018;56:61–67. doi: 10.3760/cma.j.issn.0529-5815.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Sheng W, Yuan C, Wu L, Yan J, Ge J, Lei J. Clinical application of a three-dimensional reconstruction technique for complex liver cancer resection. Surg Endosc. 2022;36:3246–3253. doi: 10.1007/s00464-021-08636-2. [DOI] [PubMed] [Google Scholar]
- 18.Jang JY, Lee JS, Kim HJ, Shim JJ, Kim JH, Kim BH, Um SH. The general rules for the study of primary liver cancer. J Liver Cancer. 2017;17:19–44. [Google Scholar]
- 19.Orcutt ST, Anaya DA. Liver resection and surgical strategies for management of primary liver cancer. Cancer Control. 2018;25:1073274817744621. doi: 10.1177/1073274817744621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer-Szary J, Luis MS, Mikulski S, Patel A, Schulz F, Tretiakow D, Fercho J, Jaguszewska K, Frankiewicz M, Pawłowska E, Targoński R, Szarpak Ł, Dądela K, Sabiniewicz R, Kwiatkowska J. The role of 3D printing in planning complex medical procedures and training of medical professionals-cross-sectional multispecialty review. Int J Environ Res Public Health. 2022;19:3331. doi: 10.3390/ijerph19063331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi S, Chen G, Cao P, Hu J, He G, Luo J, He J, Peng X. Safety and efficacy of enhanced recovery after surgery (ERAS) programs in patients undergoing hepatectomy: a prospective randomized controlled trial. J Clin Lab Anal. 2018;32:e22434. doi: 10.1002/jcla.22434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng G, Ren JK, Wang HT, Deng L, Chen ZB, Fan YW, Tang YJ, Zhang T, Tang D. Tumor burden score dictates prognosis of patients with combined hepatocellular cholangiocarcinoma undergoing hepatectomy. Front Oncol. 2023;12:977111. doi: 10.3389/fonc.2022.977111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong J, Yang S, Zeng J, Cai S, Ji W, Duan W, Huang Z. Precision in liver surgery[C]//Seminars in liver disease. Thieme Medical Publishers. 2013;33:189–203. doi: 10.1055/s-0033-1351781. [DOI] [PubMed] [Google Scholar]
- 24.Wong-Lun-Hing EM, Van Dam RM, Heijnen LA, Busch OR, Terkivatan T, van Hillegersberg R, Slooter GD, Klaase J, de Wilt JH, Bosscha K, Neumann UP, Topal B, Aldrighetti LA, Dejong CH. Is current perioperative practice in hepatic surgery based on enhanced recovery after surgery (ERAS) principles? World J Surg. 2014;38:1127–1140. doi: 10.1007/s00268-013-2398-6. [DOI] [PubMed] [Google Scholar]
- 25.Wen H, Dong JH, Zhang JH, Duan WD, Zhao JM, Liang YR, Shao YM, Ji XW, Tai QW, Li T, Gu H, Tuxun T, He YB, Huang JF. Ex vivo liver resection and autotransplantation for end-stage alveolar echinococcosis: a case series. Am J Transplant. 2016;16:615–624. doi: 10.1111/ajt.13465. [DOI] [PubMed] [Google Scholar]
- 26.Morales-Navarrete H, Segovia-Miranda F, Klukowski P, Meyer K, Nonaka H, Marsico G, Chernykh M, Kalaidzidis A, Zerial M, Kalaidzidis Y. A versatile pipeline for the multi-scale digital reconstruction and quantitative analysis of 3D tissue architecture. Elife. 2015;4:e11214. doi: 10.7554/eLife.11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang R, Ma LF, Rong ZX, Li MD, Zeng JP, Wang XD, Liao HE, Dong JH. Augmented reality technology for preoperative planning and intraoperative navigation during hepatobiliary surgery: a review of current methods. Hepatobiliary Pancreat Dis Int. 2018;17:101–112. doi: 10.1016/j.hbpd.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Saito Y, Sugimoto M, Imura S, Morine Y, Ikemoto T, Iwahashi S, Yamada S, Shimada M. Intraoperative 3D hologram support with mixed reality techniques in liver surgery. Ann Surg. 2020;271:e4–e7. doi: 10.1097/SLA.0000000000003552. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Dawa J, Suolang D, Lei Y, Wang J, Basang D. The application of preoperative three-dimensional reconstruction visualization digital technology in the surgical treatment of hepatic echinococcosis in Tibet. Front Surg. 2021;8:715005. doi: 10.3389/fsurg.2021.715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavriilidis P, Edwin B, Pelanis E, Hidalgo E, de’Angelis N, Memeo R, Aldrighetti L, Sutcliffe RP. Navigated liver surgery: state of the art and future perspectives. Hepatobiliary Pancreat Dis Int. 2022;21:226–233. doi: 10.1016/j.hbpd.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Zhou KQ, Sun YF, Cheng JW, Du M, Ji Y, Wang PX, Hu B, Guo W, Gao Y, Yin Y, Huang JF, Zhou J, Fan J, Yang XR. Effect of surgical margin on recurrence based on preoperative circulating tumor cell status in hepatocellular carcinoma. EBioMedicine. 2020;62:103107. doi: 10.1016/j.ebiom.2020.103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L, Shui Y, Yu Q, Guo Y, Zhang L, Zhou X, Yu R, Lou J, Wei S, Wei Q. Narrow-margin hepatectomy resulted in higher recurrence and lower overall survival for R0 resection hepatocellular carcinoma. Front Oncol. 2021;10:610636. doi: 10.3389/fonc.2020.610636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu G, Jin B, Xian X, Yang H, Zhao H, Du S, Makuuchi M, Pawlik TM, Mao Y. Evolutions in the management of hepatocellular carcinoma over last 4 decades: an analysis from the 100 most influential articles in the field. Liver Cancer. 2021;10:137–150. doi: 10.1159/000513412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu R, Liu Q. Optimization of Interventions[M]//Clinical Decision Making for Improving Prognosis. Springer. 2022:109–149. [Google Scholar]
- 35.Hallet J, Gayet B, Tsung A, Wakabayashi G, Pessaux P 2nd International Consensus Conference on Laparoscopic Liver Resection Group. Systematic review of the use of pre-operative simulation and navigation for hepatectomy: current status and future perspectives. J Hepatobiliary Pancreat Sci. 2015;22:353–362. doi: 10.1002/jhbp.220. [DOI] [PubMed] [Google Scholar]
- 36.Chen H, He Y, Jia W. Precise hepatectomy in the intelligent digital era. Int J Biol Sci. 2020;16:365–373. doi: 10.7150/ijbs.39387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudo H, Ishizawa T, Tani K, Harada N, Ichida A, Shimizu A, Kaneko J, Aoki T, Sakamoto Y, Sugawara Y, Hasegawa K, Kokudo N. Visualization of subcapsular hepatic malignancy by indocyanine-green fluorescence imaging during laparoscopic hepatectomy. Surg Endosc. 2014;28:2504–2508. doi: 10.1007/s00464-014-3468-z. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Hong SK, Lim J, Lee JM, Cho JH, Choi Y, Yi NJ, Lee KW, Suh KS. Demarcating the exact midplane of the liver using indocyanine green near-infrared fluorescence imaging during laparoscopic donor hepatectomy. Liver Transpl. 2021;27:830–839. doi: 10.1002/lt.26019. [DOI] [PubMed] [Google Scholar]
- 39.Levesque E, Martin E, Dudau D, Lim C, Dhonneur G, Azoulay D. Current use and perspective of indocyanine green clearance in liver diseases. Anaesth Crit Care Pain Med. 2016;35:49–57. doi: 10.1016/j.accpm.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Zheng J, Xie W, Huang Y, Zhu Y, Jiang L. The technique of 3D reconstruction combining with biochemistry to build an equivalent formula of indocyanine green (ICG) clearance test to assess the liver reserve function. BMC Surg. 2020;20:283. doi: 10.1186/s12893-020-00952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


