Scheme 1. Synthesis of the Precursors 8 and 14.
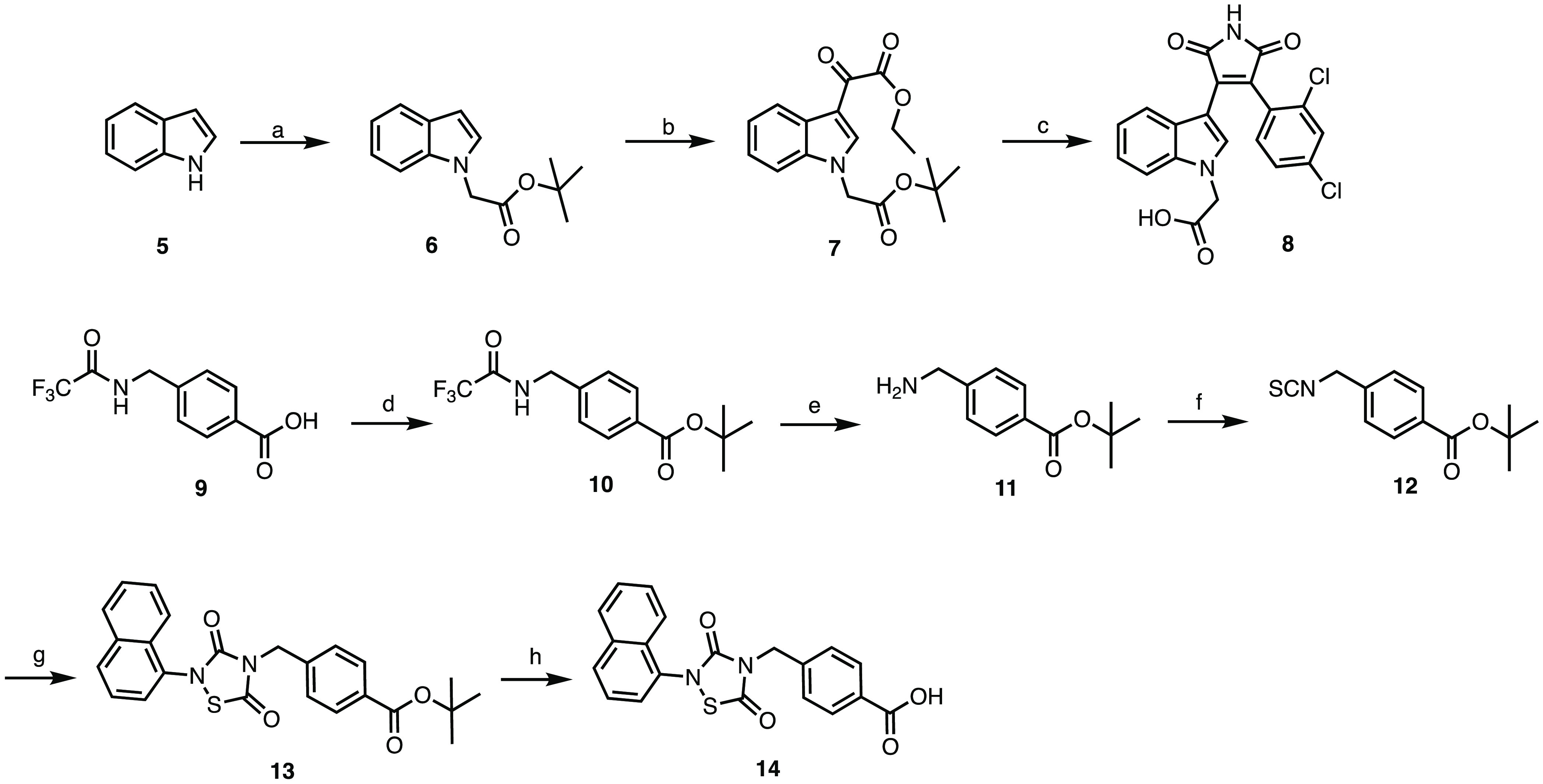
Reagents and conditions. (a) tert-butyl bromoacetate, K2CO3, acetone, reflux, 12 h, 50% yield; (b) ethyl chlorooxoacetate, diethyl ether, rt, 12 h, N2, 42% yield; (c) 2-(2,4-dichlorophenyl)acetamide, KOtBu, DMF, rt, 12 h, N2, 39% yield; (d) tert-butanol, EDCI, DMAP, THF, rt, 12 h, 77% yield; (e) K2CO3, H2O/MeOH, rt, 12 h, 79% yield; (f) 1,1′-thiocarbonyldi-2(1H)-pyridone, DCM, rt, 12 h, 55% yield; (g) naphthyl isocyanate, sulfuryl dichloride, THF, rt, 12 h, N2 then air, THF, rt, 30 min, 57% yield; (h) TFA, DCM, rt, 12 h, 81% yield.
