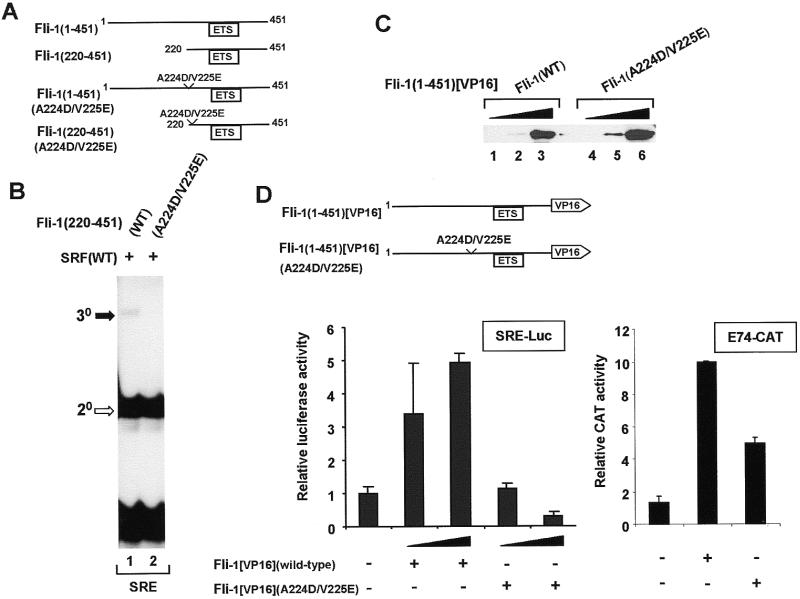
Figure 7.
Mutations in the N-terminal SBM reduce Fli-1 recruitment to the c-fos SRE in vitro and in vivo. (A) Diagrammatic representation of wild-type and mutant, full-length and truncated Fli-1 constructs. (B) Gel retardation analysis of ternary complex formation by wild-type and A224D/V225E mutant Fli-1(220–451), wild-type coreSRF and the c-fos SRE. The locations of the ternary (30, closed arrow) Fli-1–SRF–SRE and binary (20, open arrow) SRF–SRE complexes are indicated. (C) Western analysis of the expression of wild-type Fli-1(1–451)[VP16] and mutant Fli-1(1–451)[VP16](A224D/V225E) in NIH 3T3 cells. Lysates are analysed that contain increasing amounts of each Fli-expression vector (0.25 µg, lanes 1 and 4; 1 µg, lanes 2 and 5; 5 µg, lanes 3 and 6) and expression was detected using an anti-VP16 antibody. (D) Activation of SRE-luciferase (left) and E74-CAT (right) reporters by wild-type and mutant Fli-1–VP16 fusion proteins. The SRE-luciferase reporter construct (50 ng) was transfected into NIH 3T3 cells either alone or with increasing amounts of wild-type and mutant A224D/V225E Fli-1(1–451)[VP16] (0.5 and 1 µg) and a β-galactosidase expression vector. For the E74-CAT transfections, 1 µg E74-CAT and 0.5 µg of wild-type or mutant Fli-1 were cotransfected.