Table 1. Optimization of the C4-Phosphonation of 3-Cyanopyridinea.
| entry | base | oxidant | solvent | 3a, yield [%]b |
|---|---|---|---|---|
| 1 | NaHCO3 | chloranil | THF | |
| 2 | NaOH | chloranil | THF | |
| 3 | tBuOK | chloranil | THF | 79 |
| 4 | chloranil | THF | ||
| 5 | tBuOKc | chloranil | THF | |
| 6 | tBuOKd | chloranil | THF | 52 |
| 7 | tBuOKe | chloranil | THF | 17 |
| 8 | tBuOK | air | THF | |
| 9 | tBuOK | S8 | THF | |
| 10 | tBuOK | O2 | THF | |
| 11 | tBuOK | I2 | THF | |
| 12 | tBuOK | DDQ | THF | 54 |
| 13 | tBuOK | chloranil | ACN | 64 |
| 14 | tBuOK | chloranil | Et2O | 62 |
| 15 | tBuOKf | chloranil | THF | 71 |
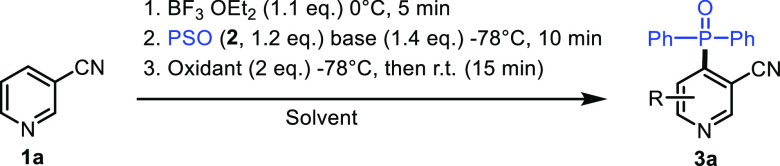
Reaction conditions: 3-cyanopyridine 1a (1 mmol, 1 equiv), BF3·OEt2 (1.1 mmol, 1.1 equiv), diphenylphosphine oxide 2a (1.2 mmol, 1.2 equiv), tBuOK (1.4 mmol, 1.4 equiv), chloranil (2 mmol), solvent (2 mL) at −78 °C, 10 min.
Isolated yield.
In the absence of BF3·OEt2.
BCl3 instead of BF3·OEt2.
0.2 equiv of BF3·OEt2.
T = −40 °C.