Figure 4.
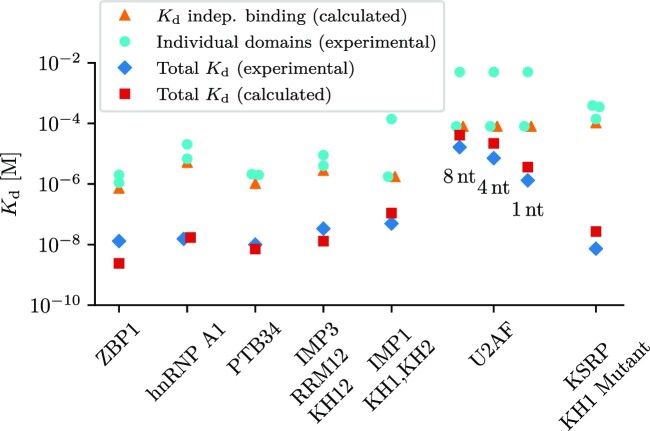
Measured avidities are in good agreement with model predictions. We found seven RBPs composed of two or three RBDs (or RBD pairs) for which dissociation constants of the full-length protein had been measured together with those of individual RBDs (4–11,39). We used the simple thermodynamic model to estimate the avidities of the full-length RBPs from those of their individual domains and from linker lengths l and protein binding site distances d and found agreement within a factor of ∼5. No free fitting parameters were used (see Supplementary Methods, section 6 for details). Orange triangles indicate the theoretical case of independent binding of the two or three domains (equivalent to an infinitely long RNA linker), calculated as the sum of Ka values of individual domains.