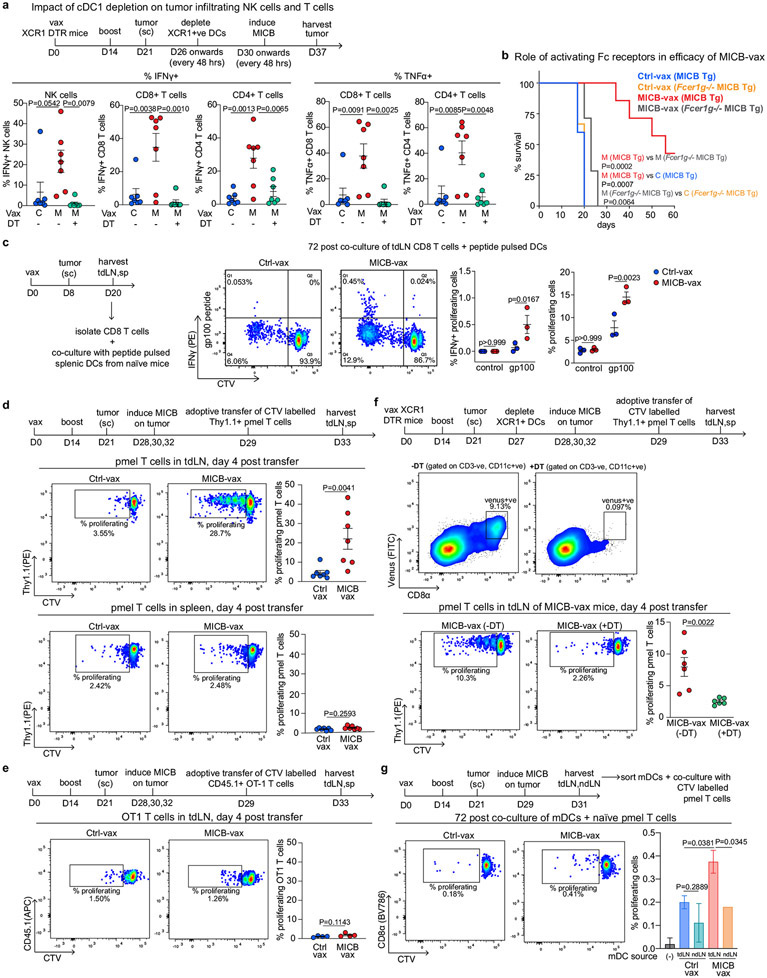
Extended Data Fig. 10 ∣. Cross-presentation of endogenous melanoma antigen by dendritic cells from MICB-vax mice.
a, Impact of cDC1 depletion on MICB vaccine-induced T-cell and NK accumulation within tumors in Xcr1DTR mice. Mice were treated +/− diphtheria toxin (DT) starting on day 26 following immunization with Ctrl-vax (C) or MICB-vax (M) (days 0 and 14) and B16F10 (MICB-dox) tumor implantation (day 21). Immune cells were analyzed in tumors 7 days following MICB induction on tumors with dox (day 37) (n = 7 mice/group). b, Contribution of activating Fc receptors to efficacy of MICB-vax. Survival curves of FceR1g−/− MICB-Tg versus MICB-Tg mice immunized with Ctrl-vax (blue) and MICB-vax (red) (n = 7 mice/group). c, Analysis of endogenous gp100 specific CD8 T-cell responses. CD8 T-cells were isolated from tdLN of mice immunized with MICB-vax or Ctrl-vax, labeled with the CTV cell proliferation dye and then co-cultured for 72 h with DCs pulsed with control (Ova) or gp100 peptide (10μg/ml). Intracellular cytokine staining (IFNγ) and CTV dilution are shown in representative flow cytometry plots (left); data are quantified for T-cells from both vaccine groups (3 mice/group, right). d, Proliferation of transferred CD8 T-cells specific for the gp100 melanoma antigen (from Pmel-1 transgenic mice) in tumor-draining lymph nodes of mice immunized with MICB-vax compared to Ctrl-vax. Mice were vaccinated twice (days 0 and 14) and B16F10 (MICB-dox) tumor cells were implanted subcutaneously on day 21. Doxycycline treatment was initiated on day 28 to induce MICB expression on tumor cells, one day prior to transfer of Thy1.1+ Pmel-1 CD8 T-cells (2x106 cells/mouse). Proliferation of CTV-labeled Pmel-1 T-cells was analyzed in tumor-draining LN (top) and spleen (bottom, control organ) four days following T-cell transfer. Cells were gated based on CD3, CD8 and Thy1.1 markers; shown is Thy1.1 marker of transferred Pmel-1 T-cells (Y-axis) and CTV dye dilution in proliferating T-cells (X-axis). Proliferating T-cell populations are indicated in representative flow plots (left) and quantification is shown (right) across the entire cohort of Ctrl-vax (blue) versus MICB-vax (red) mice (n = 7 mice/group). e, Control experiment for (d) with CD8 T-cells of irrelevant specificity (OT-1 T-cells, n = 4 mice/group). f, Role of XCR1+ DCs in the activation of transferred gp100-specific pmel-1 CD8 T-cells. Xcr1DTR mice were immunized with MICB-vax (days 0 and 14), and B16F10 (MICB-dox) melanoma cells were implanted on day 21. XCR1+ DCs were depleted by injection of diphtheria toxin (+DT, green) or solvent as a control (−DT, red) one day prior to induction of MICB expression by tumor cells with doxycycline. Thy1.1+ Pmel-1 CD8 T-cells were transferred and proliferation of these T-cells was analyzed in tdLN four days later by dilution of the CTV dye. Top flow cytometry plots show depletion of XCR1+ cells (Venus fluorescent reporter protein) in diphtheria toxin (+DT) treated mice (right) compared to control mice (−DT, left), six days following initiation of DT treatment. Bottom flow cytometry plots show proliferation of transferred pmel-1 CD8 T-cells based on dilution of the CTV dye (X-axis); data are quantified on the right (n=6 mice/group). g, Presentation of gp100 peptide by migratory DCs from MICB-vax mice. Naïve Pmel-1 CD8 T-cells were co-cultured for 72 h with migratory DCs (mDC, CD11c+, IA/E high) isolated from tdLN or non-tumor draining LN of Ctrl-vax or MICB-vax mice (pooled from 10 mice/group) implanted with B16F10 (MICB) tumor cells. CD8 T-cell proliferation was assessed based on CTV dilution. Data are representative of two independent experiments (a-d, f-g). Data from a single experiment (e). One-way ANOVA with Tukey’s multiple comparison test (a); log rank (Mantel-Cox) test (b); two-tailed Mann Whitney test (c-f); one-way ANOVA with Dunnett’s multiple comparison test (g). Data depict mean +/− SEM (a, c–f) or mean +/− SD (g).