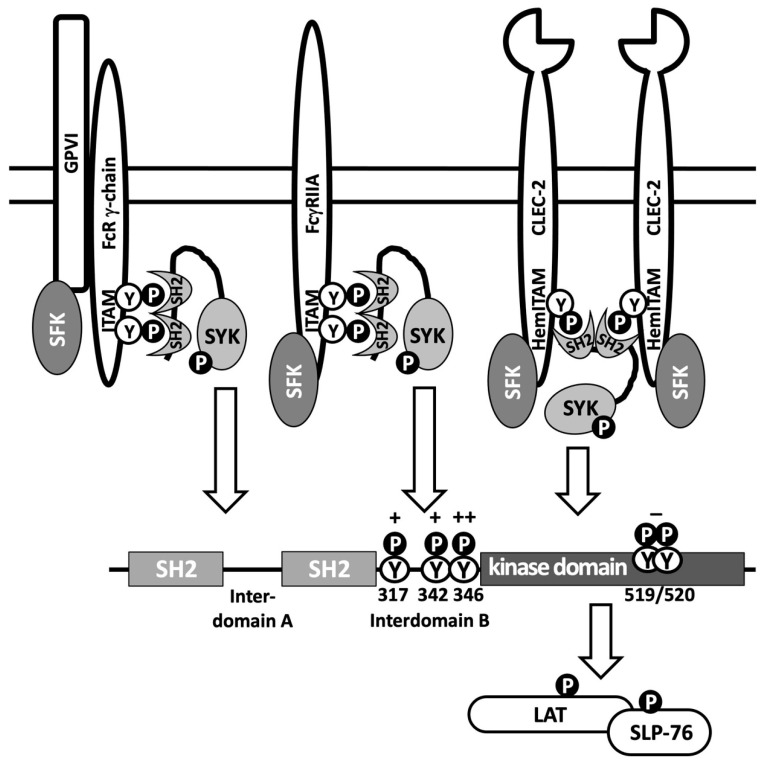
Figure 2.
Syk in signaling through ITAM- or hemITAM-bearing receptors. Major upstream events of signaling through ITAM- or hemITAM-bearing receptors are schematically represented using platelet receptors as an example. Receptor-proximal events involve Src-family protein tyrosine kinases (SFK). Syk interacts with phosphotyrosines of ITAMs and hemITAMs through its tandem SH2 domains. This interaction makes Syk tyrosine residues available for phosphorylation by SFK and autophosphorylation by Syk itself. Major regulatory tyrosines of Syk are shown. Under physiological conditions, Y346 is mostly phosphorylated by SFK, while Y317, Y342, and Y519/520 are mostly autophosphorylated. Following activation through a receptor, Syk phosphorylates its main protein substrates, LAT and SLP-76, adaptor proteins which initiate a signal transduction pathway to induce platelet responses. TULA-2 down-regulates Syk-mediated signaling by dephosphorylating several pY-sites of Syk; their sensitivity to TULA-2-dependent dephosphorylation varies from high (++) to appreciable (+) to negligible (−), as indicated. See the text for details.