Abstract
Antisense RNA was observed to elicit plant disease resistance and post-translational gene silencing (PTGS). The universal mechanism of RNA interference (RNAi) was shown to be induced by double-stranded RNA (dsRNA), an intermediate produced during virus replication. Plant viruses with a single-stranded positive-sense RNA genome have been instrumental in the discovery and characterization of systemic RNA silencing and suppression. An increasing number of applications for RNA silencing have emerged involving the exogenous application of dsRNA through spray-induced gene silencing (SIGS) that provides specificity and environmentally friendly options for crop protection and improvement.
Keywords: RNAi, immunity, HIGS, VIGS, SIGS
1. Introduction
RNA silencing is a revolutionary innate immunity mechanism in eukaryotes that has greatly expanded our knowledge of gene expression and regulation in plants. RNA interference (RNAi) is an important regulatory mechanism that has become an invaluable tool for plant research, especially in terms of understanding the effects of gene regulation in response to abiotic and biotic stress. RNAi has enabled researchers to gain insight into gene function, pest resistance, and physiological processes in plants. Although RNA is known to play critical roles in biology, the extensive capabilities and complexity of this nucleic acid remained elusive and not fully understood. The intrinsic nature of the relatively labile, often single-stranded RNA molecule, and limited availability of RNA-dependent enzymes had slowed characterization and progress. Traditional research focused on messenger transcript (mRNA), transfer RNA (tRNA), and ribosomal RNA (rRNA), but the universality of the molecule to life remained underestimated [1]. The occurrence of viruses with RNA genomes (gRNA), the dominant genome type of viruses in plants, provided an extraordinary platform for the study of RNA function and gene expression. In a relatively brief period of time, knowledge of the dynamic RNA molecule has dramatically increased, and our understanding of RNA capabilities and applications rapidly expanded. This review summarizes key developments in the discovery and characterization of RNAi and examines applications in plants to advance agricultural biotechnology, crop engineering, pest control, and virus resistance.
Plant viruses can cause major diseases in crops worldwide. Management has relied on a combination of strategies involving virus eradication and transmission prevention. Early studies reported the occurrence of cross-protection in plants where infection with a mild strain of a virus protected against infection by more pathogenic strains [2]. A major advance based on this phenomenon, and the suggestion that introduction of the viral coat protein may provide protection, was the resistance observed with the introduction of the Tobacco mosaic virus (TMV) coat protein mRNA into transgenic tobacco [3]. Several subsequent studies reported a similar resistance for other groups of plant single-stranded RNA (ssRNA) viruses including the family Solemoviridae, one of the most devastating group of viruses worldwide for many important crops [4,5]. Molecular characterization and control of Solemoviridae has been especially challenging as they are phloem-limited and transmitted in a persistent circulative manner by specific aphids.
2. RNA Interference
Remarkably, transformation of plants with the genus Polerovirus coat protein antisense RNA of the Potato leafroll virus produced similarly high levels of reduced virus titre and disease resistance as the corresponding sense mRNA [4,6]. The response was rapid and all transformed plants exhibited sequence-specific sustained high levels of immunity regardless of the virus inoculum concentration (Figure 1). Vector transmission of the virus by the green peach aphid Myzus persicae was reduced and disease symptoms in foliage and tubers were eliminated. This showed that RNA was capable of conferring resistance as a trigger molecule and was subsequently observed in other plant virus groups [7]. Replicative intermediates of RNA viruses include double-stranded RNA (dsRNA) and dsRNA secondary structures that are produced to regulate gene expression and are relatively stable compared to ssRNA due to the widespread occurrence of resilient ssRNA ribonucleases [8]. The disease resistance achieved with antisense RNA demonstrated an inherent ability of RNA to protect against pathogens.
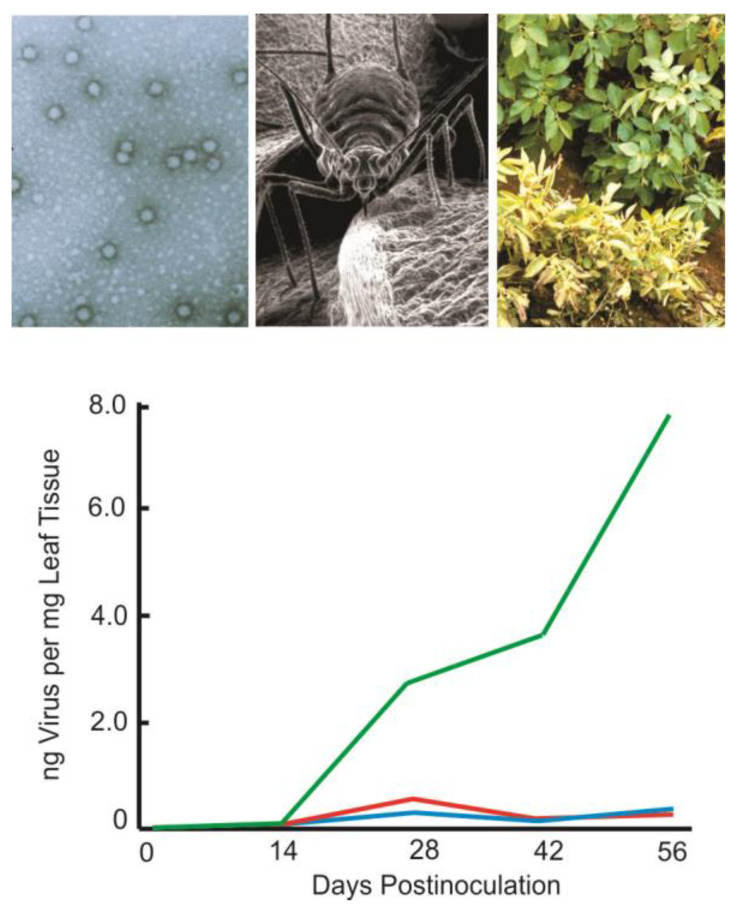
Figure 1.
RNA interference (RNAi) against virus infection. Sense and antisense RNA protection against the single-stranded positive-sense RNA Potato leafroll virus (PLRV). Although phloem limited, members of the genus Polerovirus are transmitted in a persistent, non-propagative manner and cause considerable disease-related losses worldwide. The icosahedral virus is approximately 25 nm in size (top left transmission electron photomicrograph) is transmitted in an aphid-specific manner by the green peach aphid, Myzus persicae, approximately 2.5 mm in length (top middle scanning electron photomicrograph). Disease symptoms include stunting and chlorosis of infected plants (top right) that reduce yield and quality. Virus titres in plants expressing coat protein messenger RNA (mRNA, red line) or antisense RNA (aRNA, blue line) reduced virus levels significantly as compared to untransformed controls (green line), determined by double antibody sandwich (DAS) enzyme-linked immunosorbent assay (ELISA).
Experiments to transiently or stably increase endogenous gene expression often unexpectedly produced a decrease in mRNA. For example, attempts to overexpress chalcone synthase (CHS) in pigmented petunia petals blocked anthocyanin biosynthesis [9]. Developmental timing and expression of the CHS mRNA by the endogenous gene was not altered but the level of transcript was reduced by 50 fold. This posttranslational gene silencing (PTGS) highlighted a regulatory mechanism of gene expression involving RNA interference. Polygalacturonase involved in plant cell wall degradation and ripening was inhibited in transgenic tomato expressing antisense RNA [10]. Similarities between viral defense and gene silencing mechanisms suggested a common innate immunity in plants, including the systemic signalling in gene silencing contributing to the sequence-specific RNA interference [11,12].
3. Characterization of RNA Interference
RNA interference (RNAi) involves a sequence-specific suppression of gene expression by transcriptional or translational repression. The results of the RNAi characterization demonstrated that feeding or injecting gene-specific dsRNA into Caenorhabditis elegans resulted in the disappearance of the targeted message [13]. Silencing effects were observed with only a few molecules of unc-22 dsRNA per cell supporting a role as a trigger molecule. The RNAi mechanism is a naturally occurring process in most eukaryotes, conferring an ability of dsRNA to induce a sequence-specific systemic silencing process [6,9,10,13].
Exogenous dsRNA initiates RNAi by activating the ribonuclease Dicer enzymes that bind and cleave dsRNA into 21–24-base-pair small interfering RNA (siRNA) fragments with 3′ overhangs of 2–3 nucleotides (Figure 2). Dicer proteins have an RNA helicase domain, RNase III motifs, and nucleic-acid-binding PAZ domain [7]. The siRNA is converted to ssRNA when the sense complimentary RNA strand is degraded by Argonaute (AGO) enzymes and the antisense guide strand is incorporated into the RNA-induced silencing complex (RISC). Members of AGO possess a PAZ domain and a PIWI domain, resembling RNaseH, that are required for cleavage activity [7]. The RISC complex further uses this strand to bind and degrade additional copies of sense complimentary RNA. Systemic silencing occurs and the inherit specificity suggests that nucleic acid is the signal molecule in plants [7,12]. Amplification of even weak silencing signals indicates that RNA-dependent RNA-polymerase (RDRP) recognition and replication elicits effective silencing.
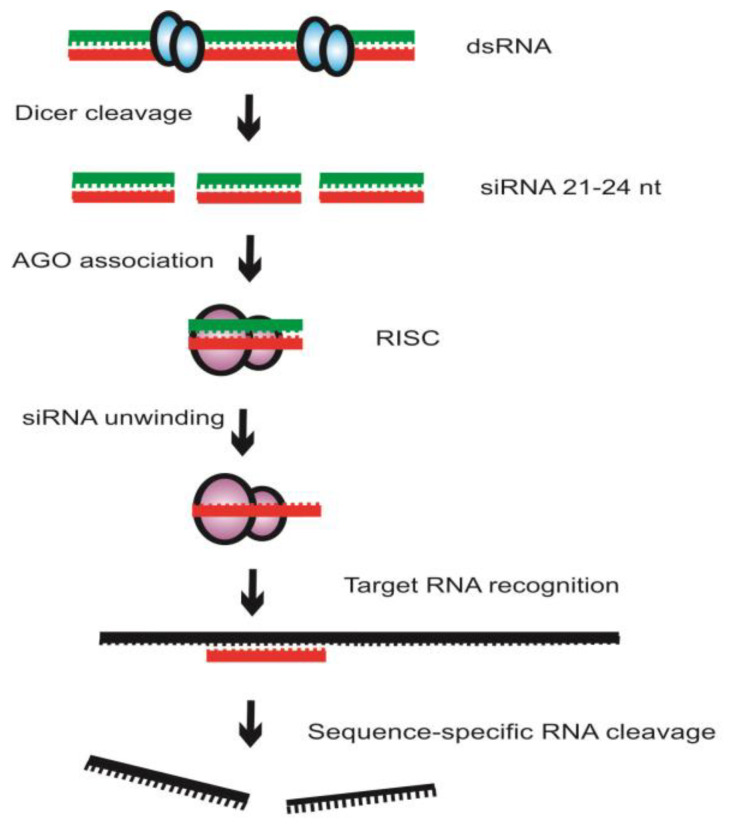
Figure 2.
Mechanism of RNA interference (RNAi). RNAi is initiated by the enzyme Dicer that cleaves double-stranded RNA (dsRNA) into short fragments of approximately 21- to 24-nucleotide short interfering RNA (siRNA). The siRNA is unwound into single-stranded RNA and the sense RNA (green) is further cleaved and degraded by the enzyme Argonaute (AGO). The antisense RNA (red) is recruited into the RNA-induced silencing complex (RISC) that binds to the target sense RNA through the specificity of the complementary antisense RNA.
The occurrence of double-stranded RNA during viral RNA replication and hairpin RNA secondary structures regulating gene expression, indicated that ssRNA viruses have an inherent protective mechanism from RNAi [14,15]. Silencing suppressors were subsequently identified within RNA virus genomes that targeted different components of RISC, such as the DICER-LIKE (DCL) proteins, and inhibit innate RNA silencing [16]. Similar to the systemic nature of RNAi, silencing suppressors were also capable of systemic silencing suppression [17]. The application of RNA silencing suppressors, such as the Tomato bushy stunt tombusvirus p19 protein, are often required in preventing PTGS in plant studies expressing homologous or heterologous genes [14].
4. Applications of RNA Interference
Stably or transiently expressed genes and nucleic acids in genetically engineered plants is often utilized in the study of gene function or the heterologous production of commercially valuable products. The use of full-length infectious clones (FLICs) of RNA viruses has facilitated the amplification of targeted genes, providing a convenient vector platform that can circumvent RNAi for site-directed mutations and increase or reduce gene expression to characterize PTGS and produce valuable heterologous commercial products. Application of virus-induced gene silencing (VIGS) has successfully utilized several RNA virus vectors including Tobacco rattle virus (TRV), Potato virus Y (PVY), TMV, and PLRV [18,19,20,21]. Different virus vectors confer specific advantages such as titre and tissue specificity. For example, field-grown plants are subjected to strict containment by regulatory agencies to limit unexpected transmission in the environment by vectors. Phloem-specific expression by the PLRV FLIC is not transmitted mechanically or by vector when the capsid readthrough protein is replaced by the heterologous nucleic acid, eliminating accidental movement of genetic materials (Figure 3).
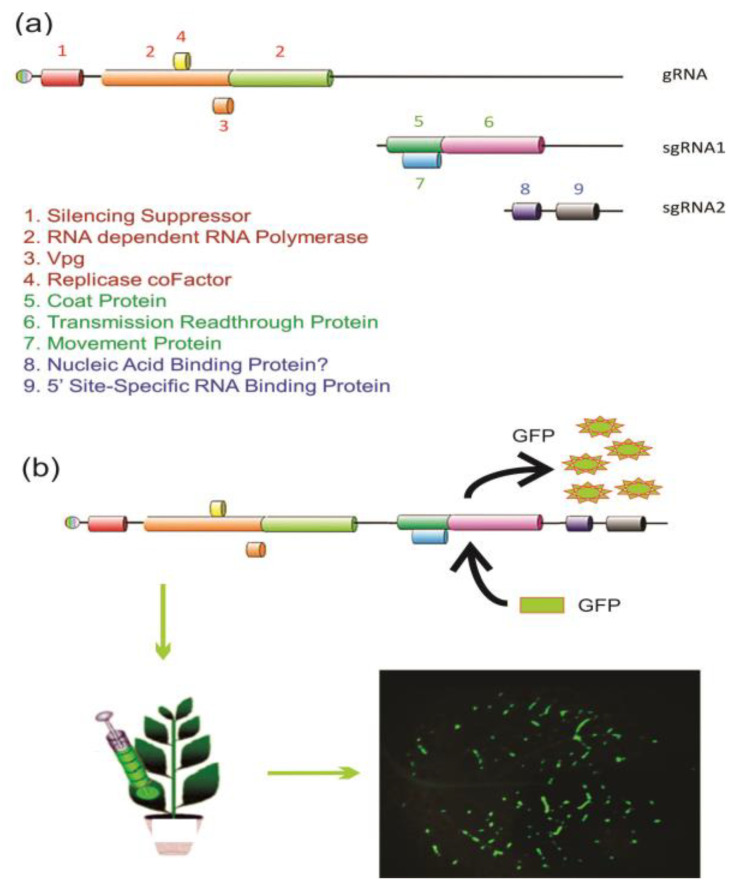
Figure 3.
RNA virus replication and applications. (a) Genomic and subgenomic RNA for the replication and translational strategies of the Potato leafroll virus, including a silencing suppressor produced immediately following virus disassembly. Replication involves the production of antisense RNA and subsequent sense subgenomic RNAs (sgRNAs) and expression of proteins involves several translational strategies including leaky start and stop codons, proteolytic site-specific cleavage of genomic RNA (gRNA), and an internal ribosomal entry site (IRES) sequence. (b) A full-length infectious clone (FLIC) of the Potato leafroll virus RNA amplifies expression of heterologous sequences for virus-induced gene silencing (VIGS) or production of commercially valuable proteins as shown (magnification 0.25×) with green fluorescent protein (GFP).
Innate immunity is more complex than originally envisioned and the RNAi regulatory mechanism is independent of other recognition and signalling pathways. Identification of genes for gene receptors and avirulence proteins has advanced our understanding of cellular resistance to a wide range of pathogens, including Pseudomonas syringae, Cladosporium fulvum, and Verticillium species [22,23,24,25]. Mechanisms for signal amplification and recognition by receptors of sessile plants has improved our understanding of an important component of innate immunity [26,27]. Cross-protection and intracellular communication has expanded with the discovery of RNAi and its role in innate immunity and gene regulation through extracellular plant and fungal RNA [28,29]. Together the different sources of innate immunity provide complementary strategies in controlling historically devastating crop losses and emerging new threats to food production.
Exogenously introduced dsRNA to target plant pests began with the introduction of dsRNAs through microinjections [30,31]. Microinjections are a favoured laboratory technique because incredibly precise amounts of dsRNA can be introduced into the target organism, allowing for precise delivery [32]. Although an adequate delivery method for lab- and smaller-scale applications, microinjection unfortunately is not suitable for field-level control of plant pests and pathogens [30,31]. Another delivery method involves the soaking of an organism in a suspension that contains the target dsRNA or directly spraying it with a solution containing the dsRNA [32]. This method may not be as exact as microinjection; however, it is often used because of its ease of use and overall convenience. Many other methods of RNA delivery have been examined and application choice is often influenced by several factors including efficacy and economics (Table 1).
Investigators have created transgenic plants that express desired dsRNAs to cause RNAi-induced gene silencing in the target organism when it ingests plant material, referred to as Host-Induced Gene Silencing (HIGS) [31,33]. One example of HIGS was transgenic Zea mays (corn) called SmartStax Pro that was created to target corn rootworm (Diabrotica vwirgifera virgifera) that was approved for commercial use by the U.S. Environmental Protection Agency, the U.S. Food and Drug Administration, and the U.S. Department of Agriculture [31,33,34]. Commercial acceptancet of transgenic plants has been challenging due to general public concerns related to genetic engineering, especially the stable insertion of nucleic acid from other organisms [33,35]. A similar pest control efficacy was achieved with exogenously applied dsRNA in plants, representing a friendlier environmental and regulatory strategy for protection and production improvements [30].
Microorganisms transformed to contain target dsRNA have also been evaluated as a method for exogenous application. One notable example showed that bacteria transformed to contain the target dsRNA could be fed to insects to induce RNAi [36]. These genetically modified bacteria in some cases were even able to colonize the gut of the host and continue to deliver dsRNA directly to it through the gut. Another example of the ingestion of a transformed microorganism is the study of transformed Saccharomyces cerevisiae (yeast) containing dsRNA targeting spotted wing fruit fly Drosophila suzukii [37]. This type of yeast naturally occurs on the surface of rotting fruit that D. suzukii consumes, and therefore was seen as a viable vector to induce oral ingestion of the dsRNA. They had success and found that locomotor activity, survivorship, and reproductive fitness were all negatively impacted by the complimentary dsRNA [37]. Acceptance of products derived from transgenic platforms are subjected to elevated regulatory and consumer acceptance concerns.
Table 1.
Examples of spray-induced gene silencing of different targets and organisms.
| Viral/Viroid Target | Host | dsRNA Target | Application Method | Reference |
|---|---|---|---|---|
| Sugarcane Mosaic Virus (SCMV) | Corn | Coat protein | Escherichia coli HT115 co-inoculation spray with dsRNA-producing bacteria | [38] |
| Pea Seed-borne Mosaic Virus (PSbMV) | Pea | Coat protein | dsRNA spray | [39] |
| Pepper Mild Mottle Virus (PMMoV) | Tobacco | PMMoV replicase | Spray containing dsRNA coated in Layered Double Hydroxide (LDH) bioclay | [40] |
| Tobacco, pepper | RP gene (multiple lengths) | Tobacco leaves treated with carborundum, dsRNA mixed with inoculum rubbed on leaves | [41] | |
| Tobacco | RP gene | Mechanical inoculation of dsRNA with co-inoculation using an atomizer | [42] | |
| Cucumber Mosaic Virus (CMV) | Cowpea | CMV 2b | Spray containing dsRNA coated in Layered Double Hydroxide (LDH) clay nanosheets | [40] |
| Bean Common Mosaic Virus (BCMV) | Tobacco | Nuclear inclusion b protein (Nib) and coat protein | dsRNA mechanical inoculation with carborundum sprayed with an atomizer | [43] |
| Tomato Yellow Leaf Curl Virus (TYLCV) | Tobacco, tomato | Coat protein | LDH clay nanosheets sprayed using an atomizer | [44] |
| Tobacco Mosaic Virus (TMV) | Tobacco | Coat protein and TMV p126 | dsRNA (virus co-inoculated rubbed on carborundum-dusted tobacco leaves | [45] |
| Tobacco | TMV replicase, movement protein | Tobacco leaves were dusted with celite, and dsRNA solution was rubbed on | [46] | |
| Tobacco | Coat protein | dsRNA and purified TMV solution inoculation | [47] | |
| Tobacco Etch Virus (TEV) | Tobacco | RP gene (multiple lengths) | Tobacco leaves treated with carborundum, dsRNA mixed with inoculum rubbed on leaves | [41] |
| Alfalfa Mosaic Virus (AMV) | Tobacco | RP gene (multiple lengths) | Tobacco leaves treated with carborundum, dsRNA mixed with inoculum rubbed on leaves | [41] |
| Papaya Ringspot Virus (PRSV) | Papaya | Coat protein | dsRNA in PRSV-infected papaya sap that was mechanically inoculated | [48] |
| Isolates Tirupati and Delhi | Papaya | Coat protein and HC-Pro | dsRNA in PSRV-infected papaya sap rubbed on to leaves dusted with carborundum | [49] |
| Cymbidium Mosaic Virus (CymMV) | Brassolaeliocattleya hybrida | Coat protein | Celite-treated orchid leaves were inoculated with dsRNA in the form of crude bacterial lysate, and gently rubbed onto said leaves | [50] |
| Zucchini Yellow Mosaic Virus (ZYMV) | Watermelon, cucumber, squash | Helper component proteinase (Hc-Pro) and coat protein | dsRNA in ZYMV-infected summer squash sap gently rubbed on to carborundum-dusted leaves | [51] |
| Potato Spindle Tuber Viroid (PSTVd) | Tomato | Viroid-specific gene | dsRNA rubbed on to leaves that were dusted with carborundum | [52] |
| Chrysanthemum Chlorotic Mottle Viroid (CChMVd) | Tomato and chrysanthemum | Viroid-specific gene | dsRNA rubbed on to leaves that were dusted with carborundum | [52] |
| Citrus Exocortis Viroid (CEVd) | Tomato and Gynura | Viroid-specific gene | dsRNA rubbed on to leaves that were dusted with carborundum | [52] |
| Fungal Target | Host | dsRNA Target | Application Method | Reference |
| Fusarium graminearum | Barley | Cytochrome P450 lanosterol, C-14α-demethylases CYP51A, CYP51B, CYP51C | dsRNA sprayed on detached barley leaves | [53] |
| Arabidopsis and Barley | FgCYP51A, FgCYP51B, and FgCYP51C in various combinations | Detached leaves sprayed with naked dsRNA | [54] | |
| Barley | DCL1 and DCL2, AGO1 and AGO2, AGO-interacting protein, FgQIP, RecQ helicase, several Fg RNA-dependent RNA polymerases | dsRNA sprayed on detached leaves | [55] | |
| Barley | AGO1 and AGO2, DCL1 and DCL2 in combinations | dsRNA sprayed on detached leaves | [56] | |
| Barley | CYP51A, CYP51B, CYP51C | Detached barley leaves drop-inoculated with dsRNA | [57] | |
| Fusarium asiaticum | Wheat | Myosin5 | dsRNA sprayed on plant surface | [58] |
| Wheat | Faβ2Tub-3 | Naked, sprayed dsRNA | [59] | |
| Fusarium oxysporum | Tomato | CYP51, chitin synthase 1, elongation factor 2 |
LDH nanosheet-coated dsRNA spray on leaf | [60] |
| Fusarium oxysporum f. sp. cubense | Banana | Nuclear condensin, coatomers alpha and zeta, DNA-directed RNA polymerase, ARP 2/3, cap methyltransferase, proteasome Pre4, ribosomal RNA, DNA polymerase alpha and delta subunits, adenyl cyclase, protein kinase C, FRQ RNA helicase | dsRNA aliquoted into spore suspension | [61] |
| Magnaporthe oryzae | Barley | Faβ2Tub-3 | Naked, sprayed dsRNA | [59] |
| Colletotrichum truncatum | Soybean | Faβ2Tub-3 | Naked, sprayed dsRNA | [59] |
| Botrytis cinerea | Arabidopsis, tomato, grape, rose, lettuce, onion, strawberry | Dicer-like DCL1 and DCL2 | dsRNA co-inoculated on fruits, vegetables, and rose petals | [62] |
| Cucumber | Faβ2Tub-3 | dsRNA sprayed on plant surface | [59] | |
| Grapevine | BcCYP51, BcCHS1, BcEF2 | dsRNA sprayed on plant surface | [63] | |
| Tomato, lettuce, rose, and grape | DCTN1, SAC1, DCL1, and DCL2 | Drop inoculation of dsRNA for lettuce, rose, tomato fruit, and grape, dsRNA spray for tomato leaves | [64] | |
| Rapeseed | Peroxidase activity, TIM44, thioredoxin reductase, pre-40s ribosomal particle, necrosis-inducing peptide 1 | Detached leaves sprayed with dsRNA | [65] | |
| Tomato and chickpea | DCL1 and DCL2, VPS51, bik1, and SAC1 | dsRNA spray | [66] | |
| Hyaloperonospora arabidopsis | Arabidopsis | Hpa-CesA | dsRNA and sRNA added to spore inoculation | [67] |
| Phakopsora pachyrhizi | Soybean | Acetyl-CoA acyltransferase 40S ribosomal protein S16, glycine cleavage system H protein |
Diethyl-pyrocarbonate detached leaves sprayed with dsRNA | [68] |
| Plasmopara viticola | Grapevine | PvDCL1 and pvDCL2 | dsRNA sprayed post-inoculation | [69] |
| Phytophthora infestans | Potato | Sorbitol dehydrogenase, translation elongation factor 1-a, phospholipase-D like 3, glycosylphosphatidylinositol-anchored acidic serine-threonine rich HAM34-like protein, and heat shock protein-90 |
E. coli HT115 sprayed dsRNA coated with nanoclay formation | [70] |
| Potato | Guanine-nucleotide-binding protein B subunit, haustorial membrane protein, cutinase, endo-1,3(4)-B-glucanase |
dsRNA sprayed on detached leaves | [71] | |
| Sclerotinia sclerotiorum | Barley | SsThioR, SsTlm44, SsCHC, SsAp2, SsArf72A, SsFCHO1, SsAmph, SsVATPase, and SseGFP | dsRNA clathrin-mediated endocytosis spray | [72] |
| Lettuce and collard greens | DCTN1, SAC1, DCL1, and DCL2 | Drop inoculation of dsRNA | [64] | |
| Rapeseed and Arabidopsis | Various genes involved in reactive oxygen species responses, transcription, host colonization, ribosomal biogenesis, mitochondrial protein import, and cell regulation | dsRNA sprayed on detached leaves | [65] | |
| Botryotinia fuckeliana | Strawberry | Chitin synthase class Ill, DCL1, and DCL2 | E. coli-derived minicell topical spray | [73] |
| Rhizoctonia solani | Rice | DCTN1, SAC1, and PG | Drop inoculation of dsRNA | [64] |
| Aspergillus niger | Tomato, apple, and grape | pgxB, VPS51, DCTN1, SAC1 | Drop inoculation of dsRNA | [64] |
| Verticillium dahliae | Arabidopsis | DCL1, DCL2, SAC1, and DCTN1 | Root dip co-inoculation of V. dalhiae spores and dsRNA | [64] |
| Verticillium spp. | Arabidopsis | Dicer-like DCL1 and DCL2 | dsRNA co-inoculated | [62] |
| Mycosphaerella fijiensis | Banana | Nuclear condensin, coatomers alpha and zeta, DNA-directed RNA polymerase, ARP 2/3, cap methyltransferase, proteasome Pre4, ribosomal RNA, DNA polymerase alpha and delta subunits, adenylase cyclase, protein kinase C, FRQ-interacting helicase | dsRNA spore suspension | [61] |
| Insect Target | Host | dsRNA Target | Application Method | Reference |
| Diabrotica virgifera virgifera | Corn | V-ATPase A, α-tubulin, COPI coatomer | Plant dsRNA artificial diet | [74] |
| Corn | Smooth septate junction (SSJ) | Artificial diet containing dsRNA | [75] | |
| Sitobeon avenae | Barley | Salivary sheath protein | Naked dsRNA foliar spray of leaves | [76] |
| Leptinotarsa decemlineata | Potato | Inhibitor of apoptosis, actin, HSP70, dynamin | Escherichia coli HT115 dsRNA | [77] |
| Potato | Actin | Naked dsRNA sprayed leaves | [78] | |
| Potato | Mesh gene | Naked dsRNA sprayed plants | [79] | |
| Phaedon cochleariae | Cabbage | Cactus, srp54k, rop, α-SNAP shibire, PP-α, hsc70-3, rpt3 | Naked dsRNA sprayed leaves | [78] |
| Helicoverpa armigera | Cotton | CYP6AE14, GST1 | Plant dsRNA in artificial diet | [80] |
| Chickpea | Juvenile hormone methyltransferase, acetylcholine esterase |
Chitosan nanoparticles sprayed onto plants |
[81] | |
| Cotton | CYP enzyme system | Injection of dsRNA into abdomen of fourth-instar larvae | [82] | |
| Cotton | HMG-CoA reductase | Injection of dsRNA into abdomen of 2-day-old female pupa | [83] | |
|
Henosepilachna
vigintioctopunctata |
Potato | Ecdysone receptor | Escherichia coli HT115 immersed in dsRNA and sprayed on foliage | [84] |
| Ostrinia furnaclis | Corn | CYP18A1, carboxylesterase | Plant dsRNA in artificial diet (dsRNA in leaves) | [85] |
| Plutella xylostella | Cabbage | Acetylcholine esterase genes AChE1 and AChE2 | Plants sprayed with siRNA, taken in through insect diet | [86] |
| Diaphorina citri | Citrus | CYP4C67, CYP4DA1, CYPC68, CYPG70, CYPDB1 | Insects anaesthetized and a drop of dsRNA was topically applied to the ventral side of the thorax | [87] |
| Citrus | Abnormal wing disc-like protein | On 5th-instar nymphs, a drop of dsRNA was topically applied to the ventral side of the thorax | [88] | |
| Citrus | Arginine kinase | Foliar spray, soil/root drench, tree trunk injection, and clay soaked in dsRNA added as a soil amendment to potted citrus trees, dsRNA was ingested by insects through plant material | [89] | |
| Leptinotarsa decemlineata | Potato | Actin gene | dsRNA-coated leaf surface, larvae fed for 7 days | [90] |
| Halyomorpha halys | Common bean | Juvenile hormone acid O-methyltransferase, vitellogenin | Green beans soaked in dsRNA | [89] |
| Acyrthosiphon pisum | Fava bean | Coo2 | siRNA injected into insects | [91] |
| Broadbean | Calreticulin | Injection | [92] | |
| Broadbean | Cathespin-L, vATPase | Injection and ingestion (respectively) | [93] | |
| Broadbean | Aquaporin | Ingestion | [94] | |
| Drosophila melanogaster | Broad range | vATPase | Artificial diet containing dsRNA | [91] |
| Manduca sexa | Tobacco | vATPase | Artificial diet containing dsRNA | [93] |
| Brassicogethes aeneus | Oilseed rape | Alpha COP | Dietary exposure to buds treated with dsRNA | [95] |
| Lygus lineolaris | Cotton | Inhibitor of apoptosis gene, polygalacturonase | Injection | [96] |
| Alfalfa | Polygalacturonase | Injection | [97] | |
| Nilaparvata lugens | Rice | Calreticulin, cathepsin-B, NIβ2 | Injection | [98] |
| Rice | Trehalose phosphate synthase | Ingestion | [99] | |
| Rice | vATPase subunit E | Ingestion | [100] | |
| Rice | AGO1 and Dicer | Plant dsRNA in artificial diet (leaves soaked in dsRNA) | [85] | |
| Rice | NADPH–cytochrome P450 reductase (CPR) | dsRNA injection into 3rd-instar nymphs | [101] | |
| Rice | Calmodulins NlCaM1 andNlCaM2 | dsRNA injected into nymphs | [102] | |
| Choristoneura fumiferana | Spruce | Chitin deacetylase | Injection of dsRNA into larvae and pre-pupae | [103] |
| Spodoptera exigua | Beet | Chitinase7, PGCP, chitinase1, ATPase, tubulin1, arf2, tubulin2, arf1, and helicase | Injection of dsRNA into 4th-instar larvae in the abdomen | [104] |
| Beet | SeCHSA | Fed dsRNA through artificial diet | [105] | |
| Chinese cabbage | Chitin synthase | Chinese cabbage leaf discs were soaked in guanidine coated polymer that coated dsRNA and were fed to larvae | [106] | |
| Spodoptera litura | Castor | Bt toxin receptor | dsRNA injected into early 5th-instar larvae | [107] |
| Spodoptera frugiperda | Corn | sfVATPase, sfKIF, sfCDC27 | Larvae fed dsRNA suspension | [108] |
| Sesamia nonagrioides | Corn | Juvenile hormone esterase-related gene | Inject dsRNA into 5th-instar larvae | [109] |
| Laodelphax striatellus | Rice | Cytochrome P450 monooxygenase Shadow (Sad) | dsRNA fed to 4th-instar larvae through artificial diet | [110] |
| Aphis gossypii | Cotton | Juvenile hormone-binding protein (JHBP) and vacuolar ATPase subunit H (V-ATPase-H) | dsRNA fed through artificial diet to first instar larvae | [111] |
| Aphis glycines | Soybean | TREH, ATPD, ATPE, and CHS1 | Insect orally fed on dsRNA with a nanocarrier that was sprayed on soybean plants | [112] |
| Sitobion avenae | Winter wheat | Laccase 1 | Insects fed dsRNA through artificial diet | [113] |
| Tetranychus urticae | Red kidney bean | Juvenile hormone (JH), methoprene-tolerant (Met), retinoid X receptor β, farnesoic acid O-methyltransferase, CREB-binding protein | Bean leaf discs soaked in dsRNA and fed to insects | [114] |
| Plant Target | Host | dsRNA Target | Application Method | Reference |
| Nicotiana benthamiana | Tobacco | CaMV 35S promotor | Naked dsRNA sprayed with carborundum | [115] |
| Mikana micrantha | Weeds | Chlorophyll a/b proteins | dsRNA, RNAi nanomicrosphere shRNA | [116] |
| Arabidopsis thaliana | Arabidopsis | STM and WER | Root soaking in naked dsRNA and a fluorescent nanocarrier | [117] |
| Arabidopsis | EGFP and NPTII | Naked dsRNA, suspension brushed onto leaves | [118] | |
| Arabidopsis | Mob1A, WRKY23, actin | Root soaking in dsRNA suspension | [85] | |
| Arabidopsis | CHS | Leaf infiltration of dsRNA with a carrier peptide using a syringe with no needle onto plant leaf | [119] | |
| Dendrobium hybrida | Dendrobium orchid | DhMYB1 | Rubbing plant with bacterial extract containing the dsRNA | [120] |
5. Spray-Induced Gene Silencing
Application of RNAi as a foliar spray has become of particular interest, specifically because it has promise in being turned into a viable and specific biopesticide that would be available for commercial use. This technology is referred to as Spray-Induced Gene Silencing (SIGS), and it is a method that allows non-transformative control of plant pests and pathogens [31,35]. This mechanism involves the application of long siRNAs and dsRNAs through a foliar spray to the affected host, which will induce RNAi when consumed by the target pest or pathogen. This mechanism can allow for specific control of harmful pathogens, without some of the downstream effects that a chemical-based pesticide may have on the surrounding ecosystem [35]. To date, SIGS has been successful in treating a wide range of pathogens and pests (Table 1). One major drawback of this technique is that ssRNAs and even dsRNAs are relatively unstable, especially when exposed to the elements [31,35]. Considerable effort surrounding the SIGS strategy is going into testing various coatings and methods to stabilize the RNAs to allow for higher efficiency [35].
Published exogenously applied RNA studies report efficacies of up to 100% with over 30 days of activity and were influenced by many parameters including the genetic target, RNA size, and environment (Table 1). With all of these successes, however, there are always places for improvement and many roadblocks that continue to pose challenges with SIGS. To begin with, dsRNA has relatively short environmental survival, as mentioned previously, but may be an advantage from a regulatory perspective as the molecule does not persist and contaminate the environment. Some ways of increasing this efficiency include the use of liposomes or nanoparticles as transfection agents, or even the chemical modification of one or both strands of the dsRNA [30]. Polymeric nanoparticles have been synthesized and used because of their overall stability, ease of surface modification, their biodegradability, and environmental safety. A popular polymer to use is one called chitosan, which is often used because of its relatively cheap cost, non-toxicity, and general biodegradability [30]. Taning et al. [121] reported that the use of a cationic liposome branded Lipofectamine to coat the dsRNA resulted in a 40–50% efficiency of gene-silencing. Without the use of the transfection agent, they were unsuccessful in their goal to induce gene silencing through RNAi. Chemical modifications are not often used due to their high cost and general safety concerns, but they can be used to improve molecule stability, increase double-stranded siRNA half-life in vivo, target siRNA to specific cells, and many other functions [30].
6. Conclusions
RNA silencing is an essential component of innate immunity and gene regulation in plants and a rapidly growing number of tools are available for applications. Efficacy and survivability of the dsRNA is being actively explored to meet specific environmental and industry requirements. Exogenous RNA survivability and uptake may be improved using polymeric, lipid-based, and inorganic nanoparticles (Table 1). Advances made in synthetic biology have opened new possibilities for optimizing traits by modulating metabolic and immunity pathways via siRNA delivery. Systemic cellular and plant movement of triggers and signals observed with the RISC response in a plant facilitates protection in tissues not exposed to the dsRNA [12,17]. As research continues on this fascinating technology, new possibilities for applications in agricultural improvements continue to emerge, such as creating improved crops that have higher yields and greater nutritional value, resistant to pests and disease, resilient to environment and climate changes, and higher yields and quality. Future developments are expected in the application of RNAi technology in plants and subsequently other biological organisms. For instance, research into controlling epigenetic elements in gene silencing will have important implications for both agricultural biotechnology and fundamental evolutionary studies [122]. There are still many exciting possibilities for future development and applications of RNA interference in the areas of crop improvement, pest management, and human health care therapies.
Author Contributions
Writing and original draft preparation, S.K., L.K. and M.K.; writing-review and editing, L.K. and M.K.; proofreading, L.K. and M.K.; conceptualization, L.K. and M.K.; supervision, M.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by Ontario Ministry of Food and Agricultural Affairs (OMAFRA) Alliance Project Number 030712 (M.K.) and a University of Guelph Raymond Chyc Scholarship (S.K.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gheysen G., Vanholme B. RNAi from plants to nematodes. Trends Biotechnol. 2007;25:89–92. doi: 10.1016/j.tibtech.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Wingard S.A. Hosts and symptoms of ring spot, a virus disease of plants. J. Agric. Res. 1928;37:127–153. [Google Scholar]
- 3.Abel P.P., Nelson R.S., De B., Hoffmann N., Rogers S.G., Fraley R.T., Beachy R.N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science. 1986;232:738–743. doi: 10.1126/science.3457472. [DOI] [PubMed] [Google Scholar]
- 4.Kawchuk L.M., Martin R.R., McPherson J. Resistance in transgenic potato expressing the potato leafroll virus coat protein gene. Mol. Plant Microbe Interact. 1990;3:301–307. doi: 10.1094/MPMI-3-301. [DOI] [Google Scholar]
- 5.Wilson T.M. Strategies to protect crop plants against viruses: Pathogen-derived resistance blossoms. Proc. Natl. Acad. Sci. USA. 1993;90:3134–3141. doi: 10.1073/pnas.90.8.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawchuk L.M., Martin R.R., McPherson J. Sense and antisense RNA-mediated resistance to potato leafroll virus in Russet Burbank potato plants. Mol. Plant Microbe Interact. 1991;4:247–253. doi: 10.1094/MPMI-4-247. [DOI] [Google Scholar]
- 7.Waterhouse P.M., Wang M.B., Lough T. Gene silencing as an adaptive defence against viruses. Nature. 2001;411:834–842. doi: 10.1038/35081168. [DOI] [PubMed] [Google Scholar]
- 8.Jaag H.M., Kawchuk L., Rohde W., Fischer R., Emans N., Prüfer D. An unusual internal ribosomal entry site of inverted symmetry directs expression of a potato leafroll polerovirus replication-associated protein. Proc. Natl. Acad. Sci. USA. 2003;100:8939–8944. doi: 10.1073/pnas.1332697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Krol A.R., Lenting P.E., Veenstra J., van der Meer I.M., Koes R.E., Gerats A.G.M., Mol J.N.M., Stuitje A.R. An anti-sense chalcone synthase gene in transgenic plants inhibits flower pigmentation. Nature. 1988;333:866–869. doi: 10.1038/333866a0. [DOI] [Google Scholar]
- 10.Smith C.J.S., Watson C.F., Ray J., Bird C.R., Morris P.C., Schuch W., Grierson D. Antisense RNA inhibition of polygalacturonase gene expression in transgenic tomatoes. Nature. 1988;334:724–726. doi: 10.1038/334724a0. [DOI] [Google Scholar]
- 11.Ratcliff F., Harrison B.D., Baulcombe D.C. A similarity between viral defense and gene silencing in plants. Science. 1997;276:1558–1560. doi: 10.1126/science.276.5318.1558. [DOI] [PubMed] [Google Scholar]
- 12.Voinnet O., Baulcombe D.C. Systemic signalling in gene silencing. Nature. 1997;389:553. doi: 10.1038/39215. [DOI] [PubMed] [Google Scholar]
- 13.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Science. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 14.Voinnet O., Pinto Y.M., Baulcombe D.C. Suppression of gene silencing: A general strategy used by diverse DNA and RNA viruses of plants. Proc. Natl. Acad. Sci. USA. 1999;96:14147–14152. doi: 10.1073/pnas.96.24.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anandalakshmi R., Pruss G.J., Ge X., Marathe R., Mallory A.C., Smith T.H., Vance V.B. A viral suppressor of gene silencing in plants. Proc. Natl. Acad. Sci. USA. 1998;95:13079–13084. doi: 10.1073/pnas.95.22.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fusaro A.F., Correa R.I., Nakasugi K., Jackson C., Kawchuk L., Vaslin M.F.S., Waterhouse P.M. The Enamovirus P0 protein is a silencing suppressor which inhibits local and systemic RNA silencing through AGO1 degradation. Virology. 2012;426:178–187. doi: 10.1016/j.virol.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 17.Fusaro A.F., Barton D.A., Nakasugi K., Jackson C., Kalischuk M.L., Kawchuk L.M., Vaslin M.F.S., Correa R.L., Waterhouse P.M. The luteovirus P4 movement protein is a suppressor of systemic RNA silencing. Viruses. 2017;9:294. doi: 10.3390/v9100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratcliff F., Martin-Hernandez A.M., Baulcombe D.C. Technical Advance. Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J. 2001;25:237–245. doi: 10.1046/j.0960-7412.2000.00942.x. [DOI] [PubMed] [Google Scholar]
- 19.Kumagai M.H., Donson J., Della-Cioppa G., Harvey D., Grill L.K. Cytoplasmic inhibition of carotenoid biosynthesis with Virus-derived RNA. Proc. Natl. Acad. Sci. USA. 1995;92:1679–1683. doi: 10.1073/pnas.92.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz M.T., Voinnet O., Baulcombe D.C. Initiation and maintenance of virus-induced gene silencing. Plant Cell. 1998;10:937–946. doi: 10.1105/tpc.10.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawchuk L., Jaag H.M., Toohey K., Martin R., Rohde W., Prüfer D. In planta agroinfection by Canadian and German Potato leafroll virus full-length cDNAs. Can. J. Plant. Path. 2002;24:239–243. doi: 10.1080/07060660309507002. [DOI] [Google Scholar]
- 22.Martin G.B. Map-based cloning of a protein-kinase gene conferring disease resistance in tomato. Science. 1993;262:1432. doi: 10.1126/science.7902614. [DOI] [PubMed] [Google Scholar]
- 23.Jones D.A., Thomas C.M., Hammond-Kosack K.E., Balint-Kurti P.J., Jones J.D.G. Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science. 1994;266:789–793. doi: 10.1126/science.7973631. [DOI] [PubMed] [Google Scholar]
- 24.Kawchuk L.M., Hachey J., Lynch D.R., Kulcsar F., Van Rooijen G., Waterer D.R., Robertson A., Kokko E., Byers R., Howard R.J., et al. Tomato Ve disease resistance genes encode cell surface-like receptors. Proc. Natl. Acad. Sci. USA. 2001;98:6511–6515. doi: 10.1073/pnas.091114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Förderer A., Li E., Lawson A.W., Deng Y., Sun Y., Logemann E., Zhang X., Wen J., Han Z., Chang J., et al. Wheat resistosome defines common principles of immune receptor channels. Nature. 2022;610:532–539. doi: 10.1038/s41586-022-05231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalischuk M., Müller B., Fusaro A.F., Wijekoon C.P., Waterhouse P.M., Prüfer D., Kawchuk L. Amplification of cell signaling and disease resistance by an immunity receptor Ve1Ve2 heterocomplex in plants. Comm. Biol. 2022;5:497. doi: 10.1038/s42003-022-03439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kourelis J., Marchal C., Posbeyikian A., Harant A., Kamoun S. NLR immune receptor–nanobody fusions confer plant disease resistance. Science. 2023;379:934. doi: 10.1126/science.abn4116. [DOI] [PubMed] [Google Scholar]
- 28.Weiberg A., Wang M., Lin F.M., Zhao H., Zhang Z., Kaloshian I., Huang H.D., Jin H. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science. 2013;342:118–123. doi: 10.1126/science.1239705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai Q., Qiao L., Wang M., He B., Lin F.M., Palmquist J., Huang S.D., Jin1 H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science. 2018;360:1126–1129. doi: 10.1126/science.aar4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joga M.R., Zotti M.J., Smagghe G., Christiaens O. RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: What we know so far. Front. Physiol. 2016;7:553. doi: 10.3389/fphys.2016.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sang H., Kim J.I. Advanced strategies to control plant pathogenic fungi by host-induced gene silencing (HIGS) and spray-induced gene silencing (SIGS) Plant Biotechnol. Rep. 2020;14:1–8. doi: 10.1007/s11816-019-00588-3. [DOI] [Google Scholar]
- 32.Yu N., Christiaens O., Liu J., Niu J., Cappelle K., Caccia S., Huvenne H., Smagghe G. Delivery of dsRNA for RNAi in insects: An overview and future directions. Insect Sci. 2013;1:4–14. doi: 10.1111/j.1744-7917.2012.01534.x. [DOI] [PubMed] [Google Scholar]
- 33.Dalakouras A., Wassenegger M., Dadami E., Ganopoulos I., Pappas M.L., Papadopoulou K. Genetically modified organism-free RNA interference: Exogenous application of RNA molecules in plants. Plant Physiol. 2020;182:38–50. doi: 10.1104/pp.19.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon K.H.J., Waterhouse P.M. RNAi for insect-proof plants. Nat. Biotechnol. 2007;25:1231–1232. doi: 10.1038/nbt1107-1231. [DOI] [PubMed] [Google Scholar]
- 35.Puyam A., Kaur K. Exploiting RNA interference mechanism in plants for disease resistance. In: Ul Haq I., Ijaz S., editors. Plant Disease Management Strategies for Sustainable Agriculture through Traditional and Modern Approaches. Sustainability in Plant and Crop Protection. Volume 13. Springe; Cham, Switzerland: 2020. pp. 217–236. [DOI] [Google Scholar]
- 36.Murphy K.A., West J.D., Kwok R.S., Chiu J.C. Accelerating research on Spotted Wing Drosophila management using genomic technologies. J. Pest Sci. 2016;89:631–641. doi: 10.1007/s10340-016-0741-z. [DOI] [Google Scholar]
- 37.Murphy K.A., Tabuloc C.A., Cervantes K.R., Chiu J.C. Ingestion of genetically modified yeast symbiont reduces fitness of an insect pest via RNA interference. Sci. Rep. 2016;6:22587. doi: 10.1038/srep22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gan D., Zhang J., Jiang H., Jiang T., Zhu S., Cheng B. Bacterially expressed dsRNA protects maize against SCMV infection. Plant Cell Rep. 2010;29:1261–1268. doi: 10.1007/s00299-010-0911-z. [DOI] [PubMed] [Google Scholar]
- 39.Safarova D., Brazda P., Navratil M. Incidence, distribution and molecular characterization of Pea seed-borne mosaic virus (PSbMV) in Egypt. Czech J. Genet. Plant Breed. 2014;50:105–108. doi: 10.17221/120/2013-CJGPB. [DOI] [Google Scholar]
- 40.Mitter N., Worrall E.A., Robinson K.E., Li P., Jain R.G., Taochy C., Fletcher S.J., Caroll B.J., Lu G.Q., Zu Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants. 2017;3:1607. doi: 10.1038/nplants.2016.207. [DOI] [PubMed] [Google Scholar]
- 41.Tenllado F., Díaz-Ruíz J.R. Double-stranded RNA-mediated interference with plant virus infection. J. Virol. 2001;76:12288–12297. doi: 10.1128/JVI.75.24.12288-12297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tenllado F., Martínez-García B., Vargas M., Díaz-Ruíz J.R., Ramón J. Crude extracts of bacterially expressed dsRNA can be used to protect plants against virus infections. BMC Biotechnol. 2003;3:3. doi: 10.1186/1472-6750-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Worrall E.A., Bravo-Cazar A., Nilon A.T., Fletcher S.J., Robinson K.E., Carr J.P., Mitter N. Exogenous application of RNAi-inducing double-stranded RNA inhibits aphid-mediated transmission of a plant virus. Front. Plant Sci. 2019;10:265. doi: 10.3389/fpls.2019.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Q., Li Y., Xu K., Li D., Hu H., Zhou F., Song P., Yu Y., Wei Q., Liu Q., et al. Clay nanosheet-mediated delivery of recombinant plasmids expressing artificial miRNAs via leaf spray to prevent infection by plant DNA viruses. Hortic. Res. 2020;7:179. doi: 10.1038/s41438-020-00400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konakalla N.C., Kaldis A., Berbate M., Masarapu H., Voloudakies A.E. Exogenous application of double-stranded RNA molecules from TMV p126 and CP genes confers resistance against TMV in tobacco. Planta. 2016;244:961–969. doi: 10.1007/s00425-016-2567-6. [DOI] [PubMed] [Google Scholar]
- 46.Niehl A., Soinen M., Poranen M.M., Heinlein M. Synthetic biology approach for plant protection using dsRNA. Plant Biotechnol. J. 2018;16:1679–1687. doi: 10.1111/pbi.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin G., Sun Z., Liu N., Song Y., Zhu C., Wen F. Production of double-stranded RNA for interference with TMV infection utilizing a bacterial prokaryotic expression system. App. Microbiol. Biotechnol. 2009;84:323–333. doi: 10.1007/s00253-009-1967-y. [DOI] [PubMed] [Google Scholar]
- 48.Shen W., Yang G., Chen Y., Yan P., Tue D., Li X., Zhou P. Resistance of non-transgenic papaya plants to papaya ringspot virus (PRSV) mediated by intron-containing hairpin dsRNAs expressed in bacteria. Acta Virol. 2014;58:261–266. doi: 10.4149/av_2014_03_261. [DOI] [PubMed] [Google Scholar]
- 49.Valdamundi T., Patil B.L., Kaldis A., Sai Gopal D.V.R., Mishra R., Berbati M., Voloudakis A. DsRNA-mediated protection against two isolates of Papaya ringspot virus through topical application of dsRNA in papaya. J. Virol. Methods. 2020;275:113750. doi: 10.1016/j.jviromet.2019.113750. [DOI] [PubMed] [Google Scholar]
- 50.Lau S.E., Mazumdar P., Hee T.W., Song A.L.A., Harikrishna J.A. Crude extracts of bacterially-expressed dsRNA protect orchid plants against Cymbidium mosaic virus during transplantation from in vitro culture. J. Hort. Sci. Biotechnol. 2014;89:569–576. doi: 10.1080/14620316.2014.11513122. [DOI] [Google Scholar]
- 51.Kaldis A., Berbati M., Melita O., Reppa C., Holeva M., Otten P., Voloudakis A. Exogenously applied dsRNA molecules deriving from the Zucchini yellow mosaic virus (ZYMV) genome move systemically and protect cucurbits against ZYMV. Mol. Plant Pathol. 2018;19:883–895. doi: 10.1111/mpp.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carbonell A., Martínez de Alba Á.E., Flores R., Gago S. Double-stranded RNA interferes in a sequence-specific manner with the infection of representative members of the two viroid families. Virology. 2008;371:44–53. doi: 10.1016/j.virol.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 53.Koch A., Biedenkopf D., Furch A., Weber L., Rossbach O., Abdellatef E., Linicus L., Johannsmeier J., Jelonke L., Goesmann A., et al. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathalog. 2016;12:e1005901. doi: 10.1371/journal.ppat.1005901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koch A., Hofle L., Werner B.T., Imani J., Schmidt A., Jelonek L., Kogel K.H. SIGS vs. HIGS: A study on the efficacy of two dsRNA delivery strategies to silence Fusarium FgCYP51 genes in infected host and non-host plants. Mol. Plant Pathol. 2019;20:1636–1644. doi: 10.1111/mpp.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaffar F.Y., Koch A. Catch me if you can! RNA silencing-based improvement of antiviral plant immunity. Viruses. 2019;11:673. doi: 10.3390/v11070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Werner B.T., Gaffar F.Y., Schuemann J., Biedenkopf D., Koch A.M. RNA-Spray-Mediated Silencing of Fusarium graminearum AGO and DCL Genes Improve Barley Disease Resistance. Front. Plant Sci. 2020;11:476. doi: 10.3389/fpls.2020.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofle L., Biedenkopf D., Werner B.T., Shrestha A., Jelonek L., Koch A. Study on the efficiency of dsRNAs with increasing length in RNA-based silencing of the Fusarium CYP51 genes. RNA Biol. 2020;17:463–473. doi: 10.1080/15476286.2019.1700033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song X.S., Gu K.X., Duan X.X., Xiao X.M., Hou Y.P., Duan Y.B., Wang J.X., Yu N., Zhou M.G. Secondary amplification of siRNA machinery limits the application of spray-induced gene silencing. Mol. Plant Pathol. 2018;19:2543–2560. doi: 10.1111/mpp.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu K.X., Song X., Xiao X.M., Duan X.X. A β-tubulin dsRNA derived from Fusarium asiaticum confers plant resistance to multiple phytopathogens and reduces fungicide resistance. Pest. Biochem. Physiol. 2019;153:36–46. doi: 10.1016/j.pestbp.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 60.Mohamed M.A., Youssef K. Topical delivery of host induced RNAi silencing by layered double hydroxide nanosheets: An efficient tool to decipher pathogenicity gene function of Fusarium crown and root rot in tomato. Physiol. Mol. Plant Pathol. 2021;115:101684. doi: 10.1016/j.pmpp.2021.101684. [DOI] [Google Scholar]
- 61.Mumbanza F.M., Kiggundu A., Tusiime G., Tushemereirwe W.K., Niblett C., Bailey A. In Vitro antifungal activity of synthetic dsRNA molecules against two pathogens of banana, Fusarium oxysporum f. sp. cubense and Mycosphaerella fijiensis. Pest Manag. Sci. 2013;69:1155–1162. doi: 10.1002/ps.3480. [DOI] [PubMed] [Google Scholar]
- 62.Wang M., Weiberg A., Lin F.M., Thomma B.P.H.J., Huang H.D., Jin H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants. 2016;2:16151. doi: 10.1038/nplants.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nerva L., Sandrini M., Gambino G., Chitarra W. Double-stranded RNAs (dsRNAs) as a sustainable tool against gray mold (Botrytis cinerea) in grapevine: Effectiveness of different application methods in an open-air environment. Biomolecules. 2020;10:200. doi: 10.3390/biom10020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiao L., Lan C., Capriotti L., Ah-Fong A., Nino Sanchez J., Hamby R., Heller J., Zhao H., Glass N.L., Judelson H.S., et al. Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotech. J. 2021;19:1756–1768. doi: 10.1111/pbi.13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McLoughlin A.G., Wytinck N., Talker P.L., Girard I.J., Rashid K.Y., de Kievit T., Fernando W.G.D., Whyard S., Belmonte M.F. Identification and application of exogenous dsRNA confers plant protection against Sclerotinia sclerotiorum and Botrytis cinerea. Sci. Rep. 2018;8:7320. doi: 10.1038/s41598-018-25434-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niño-Sánchez J., Sambasivam P.T., Sawyer A., Hamby R., Chen A., Czislowski E., Li P., Manzie N., Gardiner D.M., Ford R., et al. BioCLay prolongs RNA interference-mediate crop protection against Botrytis cinerea. J. Int. Plant. Biol. 2022;64:2187–2198. doi: 10.1111/jipb.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bilir O., Telli O., Norman C., Budak H., Hong Y., Tor M. Small RNA inhibits infection by downy mildew pathogen Hyaloperonospora arabidopsidis. Mol. Plant Pathol. 2019;20:1523–1534. doi: 10.1111/mpp.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu D., Chen Z.Y., Zhang C., Ganiger M. Reduction of Phakopsora pachyrhizi infection on soybean through host- and spray-induced gene silencing. Mol. Plant Pathol. 2020;21:794–807. doi: 10.1111/mpp.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haile Z.M., Gebremichael D.E., Capriotti L., Molesini B., Negrini F., Collina M., Sabbadini S., Mezzetti B., Baraldi E. Double-stranded RNA targeting dicer-Like genes compromises the pathogenicity of Plasmopara viticola on grapevine. Front. Plant Sci. 2021;12:667539. doi: 10.3389/fpls.2021.667539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sundaresha S., Sharma S., Bairwa A., Tomar M., Kumar R., Bhardwaj V., Jeevalatha A., Bakade R., Salaria N., Thakur K., et al. Spraying of dsRNA molecules derived from Phytophthora infestans, as an effective plant protection strategies for the management of potato late blight. Life Sci. 2021;78:3183–3192. doi: 10.1002/ps.6949. [DOI] [PubMed] [Google Scholar]
- 71.Kalyandurg P.B., Sundararajan P., Dubey M., Ghadamgahi F., Zahid M.A., Whisson S.C., Vetukuri R.R. Spray-induced gene silencing as a potential tool to control potato late blight disease. Phytopathology. 2021;111:2168–2175. doi: 10.1094/PHYTO-02-21-0054-SC. [DOI] [PubMed] [Google Scholar]
- 72.Wytinck N., Sullivan D.S., Biggar K.T., Crisostomo L., Pelka P., Belmonte M.F., Whyard S. Clathrin mediated endocytosis is involved in the uptake of exogenous double-stranded RNA in the white mold phytopathogen Sclerotinia sclerotiorum. Sci. Rep. 2020;10:12773. doi: 10.1038/s41598-020-69771-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Islam M.T., Davis Z., Chen L., Englaender J., Zomorodi S., Frank J., Bartlett K., Somers E., Carballo S.M., Kester M., et al. Minicell-based fungal RNAi delivery for sustainable crop protection. Microb. Biotechnol. 2021;14:1847–1856. doi: 10.1111/1751-7915.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baum J.A., Bogaert T., Clinton W., Heck G.R., Feldmann P., Ilagan O., Johnson S., Plaetinch G., Munyikwa T., Pleau M. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007;25:1322–1326. doi: 10.1038/nbt1359. [DOI] [PubMed] [Google Scholar]
- 75.Hu X., Steimel J.P., Kapka-Kitzman D.M., Davis-Vogel C., Richtman N.M., Mathis J.P., Nelson M.E., Lu A.L., Wu G., Gao Y. Molecular characterization of the insecticidal activity of double-stranded RNA targeting the smooth septate junction of western corn rootworm (Diabrotica virgifera virgifera) PLoS ONE. 2018;14:e0210481. doi: 10.1371/journal.pone.0210491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biedenkopf D., Will T., Knauer T., Jelonek L., Furch A.C.U., Busche T., Koch A. Systemic spreading of exogenous applied RNA biopesticides in the crop plant Hordeum vulgare. ExRNA. 2020;2:12. doi: 10.1186/s41544-020-00052-3. [DOI] [Google Scholar]
- 77.Maximo W.P.F., Howell J.L., Mogilicherla K., Basij M., Chereddy S.C.R.R., Palli S.R. Inhibitor of apoptosis is an effective target gene for RNAi-mediated control of Colorado potato beetle, Leptinotarsa decemlineata. Arch. Insect Biochem. Physiol. 2020;104:e21685. doi: 10.1002/arch.21685. [DOI] [PubMed] [Google Scholar]
- 78.Mehlhorn S., Ulrich J., Baden C.U., Buer B., Maiwald F., Lueke B., Geibel S., Bucher G., Nauen R. The mustard leaf beetle, Phaedon coclileariae, as a screening model for exogenous RNAi-based control of coleopteran pests. Pest. Biochem. Physiol. 2021;176:104870. doi: 10.1016/j.pestbp.2021.104870. [DOI] [PubMed] [Google Scholar]
- 79.Petek M., Coll A., Ferenc R., Razinger J., Gruden K. Validating the potential of double-stranded RNA targeting Colorado potato beetle mesh gene in laboratory and field trials. Front. Plant Sci. 2020;11:1250. doi: 10.3389/fpls.2020.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mao J., Zhang P., Liu C., Seng F. Co-silence of the coatomer β and v-ATPase A genes by siRNA feeding reduces larval survival rate and weight gain of cotton bollworm, Helicoverpa armigera. Pestic. Biochem. Physiol. 2015;118:71–76. doi: 10.1016/j.pestbp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 81.Kolge H., Kadam K., Galande S., Lanjekar V., Ghormade V. New frontiers in pest control: Chitosan nanoparticles-shielded dsRNA as an effective topical RNAi spray for gram podborer biocontrol. ACS Appl. Bio Mater. 2021;4:5145–5157. doi: 10.1021/acsabm.1c00349. [DOI] [PubMed] [Google Scholar]
- 82.Tang T., Zhao C., Feng X., Liu X., Qiu L. Knockdown of several components of cytochrome P450 enzyme systems by RNA interference enhances the susceptibility of Helicoverpa armigera to fenvalerate. Pest Manag. Sci. 2012;68:1501–1511. doi: 10.1002/ps.3336. [DOI] [PubMed] [Google Scholar]
- 83.Wang Z., Dong Y., Desnaux N., Niu C., Sambhara S. RNAi silencing of the HaHMG-CoA reductase gene inhibits oviposition in the Helicoverpa armigera cotton bollworm. PLoS ONE. 2013;8:e67732. doi: 10.1371/journal.pone.0067732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu J.J., Mu L.L., Kang W.N., Ze L.J., Shen C.H., Jin L., Anjum A.A., Li G.Q. RNA interference targeting ecdysone receptor blocks the larval-pupal transition in Henosepilachna vigintioctopunctata. Insect Sci. 2021;28:419–429. doi: 10.1111/1744-7917.12777. [DOI] [PubMed] [Google Scholar]
- 85.Li H., Guan R., Guo H., Miao X. New insights into an RNAi approach for plant defence against piercing-sucking and stem-borer insect pests. Plant Cell Environ. 2015;38:2277–2285. doi: 10.1111/pce.12546. [DOI] [PubMed] [Google Scholar]
- 86.Gong L., Chen Y., Hu Z., Hu M., Unver T. Testing insecticidal activity of novel chemically synthesized siRNA against Plutella xylostella under laboratory and field conditions. PLoS ONE. 2013;8:e62990. doi: 10.1371/journal.pone.0062990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Killiny N., Hajeri S., Tiwari S., Gowda S., Stelinski L.L., Hansen I.A. Double-stranded RNA uptake through topical application mediates silencing of five CYP4 genes and suppresses insecticide resistance in Diaphorina citri. PLoS ONE. 2014;9:e110536. doi: 10.1371/journal.pone.0110536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.El-Shesheny I., Hajeri S., El-Hawary I., Gowda S., Killiny N., Niedz R.P. Silencing abnormal wing disc gene of the Asian citrus psyllid, Diaphorina citri, disrupts adult wing development and increases nymph mortality. PLoS ONE. 2013;8:e65392. doi: 10.1371/journal.pone.0065392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghosh S.K.B., Hunter W.B., Park A.L., Gundersen-Rindal D.E. Double-stranded RNA oral delivery methods to induce RNA interference in phloem and plant-sap-feeding hemipteran insects. J. Vis. Exp. 2018;135:e57390. doi: 10.3791/57390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.San Miguel K., Scott J.G. The next generation of insecticides: dsRNA is stable as a foliar-applied insecticide. Pest Manag. Sci. 2015;72:801–809. doi: 10.1002/ps.4056. [DOI] [PubMed] [Google Scholar]
- 91.Mutti N.S., Parl Y., Reese J.C., Reeck G.R. RNAi knockdown of a salivary transcript leading to lethality in the pea aphid Acyrthosiphon pisum. J. Insect Sci. 2006;6:1–7. doi: 10.1673/031.006.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jaubert-Possami S., Le Trionnaire G., Bonhomme J., Chistophides G.K., Rispe C., Tagu D. Gene knockdown by RNAi in the pea aphid Acyrthosiphon pisum. BMC Biotechnol. 2007;7:63. doi: 10.1186/1472-6750-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Whyard S., Singh A.D., Wong S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem. Mol. Biol. 2009;39:824–832. doi: 10.1016/j.ibmb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 94.Shakesby A.J., Wallace I.S., Isaacs H.V., Pritchar J., Roberts D.M., Douglas A.E. A water-specific aquaporin involved in aphid osmoregulation. Insect Biochem. Mol. Biol. 2009;39:1–10. doi: 10.1016/j.ibmb.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 95.Willow J., Soonvald L., Sulg S., Kaasik R., Silva A.I., Taning C.N.T., Christiaens O., Smagghe G., Veromann E. First evidence of bud feeding-induced RNAi in a crop pest via exogenous application of dsRNA. Insects. 2020;11:769. doi: 10.3390/insects11110769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walker W.B., Allen M.L. Expression and RNA interference of salivary polygalacturonase genes in the tarnished plant bug Lygus lineolaris. J. Insect Sci. 2010;10:1–13. doi: 10.1673/031.010.14133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walker W.B., Allen M.L. RNA interference-mediated knockdown of IAP in Lygus lineolaris induced mortality in adult and pre-adult life stages. Entomol. Exp. Appl. 2011;138:83–92. doi: 10.1111/j.1570-7458.2010.01078.x. [DOI] [Google Scholar]
- 98.Liu S., Ding Z., Zhang C., Yang B., Liu Z. Gene knockdown by into-thoracic injection of double-stranded RNA in the brown planthopper Nilaparvata lugens. Insect Biochem. Mol. Biol. 2010;40:666–671. doi: 10.1016/j.ibmb.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 99.Chen J., Zhang D., Yao Q., Zhang J., Dong X., Tian H., Zhang W. Feeding-based RNA interference of a trehalose phosphate synthase gen in the brown planthopper Nilaparvata lugens. Insect Mol. Biol. 2010;19:777–786. doi: 10.1111/j.1365-2583.2010.01038.x. [DOI] [PubMed] [Google Scholar]
- 100.Li J., Chen Q., Lin Y., Jiang T., Wu G., Hua H. RNA interference in Nilaparvata lugens (Homoptera: Delphacidae) based on dsRNA ingestion. Pest Manag. Sci. 2011;67:852–859. doi: 10.1002/ps.2124. [DOI] [PubMed] [Google Scholar]
- 101.Liu S., Liang Q.M., Zhou W.W., Jiang Y.D., Zhu Q.Z., Yu H., Zhang C.X., Gurr G.M., Zhu Z.R. RNA interference of NADPH–cytochrome P450 reductase of the rice brown planthopper, Nilaparvata lugens, increases susceptibility to insecticides. Pest Manag. Sci. 2015;71:32–39. doi: 10.1002/ps.3760. [DOI] [PubMed] [Google Scholar]
- 102.Wang W., Wan P., Lai F., Zhu T., Fu Q. Double-stranded RNA targeting calmodulin reveals a potential target for pest management of Nilaparvata lugens. Pest Manag. Sci. 2018;74:1711–1719. doi: 10.1002/ps.4865. [DOI] [PubMed] [Google Scholar]
- 103.Quan G., Ladd T., Duan J., Wen F., Doucet D., Cusson M., Krell P.J. Characterization of a spruce budworm chitin deacetylase gene: Stage- and tissue-specific expression, and inhibition using RNA interference. Insect Biochem. Mol. Biol. 2013;43:683–691. doi: 10.1016/j.ibmb.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 104.Li H., Jiang W., Zhang Z., Xing Y., Li F., Ternius O. Transcriptome analysis and screening for potential target genes for RNAi-mediated pest control of the beet armyworm, Spodoptera exigua. PLoS ONE. 2013;8:e65931. doi: 10.1371/journal.pone.0065931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tian H., Peng H., Ya Q., Chen H., Xie Q., Tang B., Zhang W., Hansen I.A. Developmental control of a Lepidopteran pest Spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS ONE. 2009;4:e6225. doi: 10.1371/journal.pone.0006225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Christiaens O., Tardajos M.G., Martinez Reyna Z.L., Dash M., Dobruel P., Smagghe G. Increased RNAi efficacy in Spodoptera exigua via the formulation of dsRNA with guanylated polymers. Front. Physiol. 2018;9:316. doi: 10.3389/fphys.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rajagopal R., Sivakumar S., Agrawal N., Malhotra P., Bhatnagar R.K. Silencing of midgut aminopeptidase N of Spodoptera litura by double-stranded RNA establishes its role as Bacillus thuringiensis toxin receptor. J. Biol. Chem. 2002;277:46849–46851. doi: 10.1074/jbc.C200523200. [DOI] [PubMed] [Google Scholar]
- 108.Parsons K.H., Mondal M.H., McCormick C.L., Flynt A.S. Guanidinium-functionalized interpolyelectrolyte complexes enabling RNAi in resistant insect pests. Biomacromolecules. 2018;19:1111–1117. doi: 10.1021/acs.biomac.7b01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kontogiannatos D., Swevers L., Maenaka K., Park E.Y., Iatrou K., Kourti A., Wicker-Thomas C. Functional characterization of a juvenile hormone esterase related gene in the moth Sesamia nonagrioides through RNA interference. PLoS ONE. 2013;8:e73834. doi: 10.1371/journal.pone.0073834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wan P.J., Jia S., Li N., Fan J.M., Li G.G. A Halloween gene shadow is a potential target for RNA-interference-based pest management in the small brown planthopper Laodelphax striatellus. Pest Manag. Sci. 2015;71:199–206. doi: 10.1002/ps.3780. [DOI] [PubMed] [Google Scholar]
- 111.Rebijith K.B., Asokan R., Ranjitha H.H., Prasad B.S.R., Krishna V., Kumar N.K.K. Diet-delivered dsRNAs for juvenile hormone-binding protein and vacuolar ATPase-H implied their potential in the management of the melon aphid (Hemiptera: Aphididae) Environ. Entymol. 2016;45:268–275. doi: 10.1093/ee/nvv178. [DOI] [PubMed] [Google Scholar]
- 112.Yan S., Qian J., Cai C., Ma Z., Li J., Yin M., Ren B., Shen J. Spray method application of transdermal dsRNA delivery system for efficient gene silencing and pest control on soybean aphid Aphis glycines. J. Pest Sci. 2020;93:449–459. doi: 10.1007/s10340-019-01157-x. [DOI] [Google Scholar]
- 113.Zhang Y., Fan J., Francis F., Chen J. Molecular characterization and gene silencing of Laccase 1 in the grain aphid, Sitobion avenae. Arch. Insect Biochem. Physiol. 2018;97:e21446. doi: 10.1002/arch.21446. [DOI] [PubMed] [Google Scholar]
- 114.Yoon J.S., Sahoo D.K., Maiti I.B., Palli S.R. Identification of target genes for RNAi-mediated control of the Two spotted Spider Mite. Sci. Rep. 2018;8:14687. doi: 10.1038/s41598-018-32742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dalakouras A., Ganopoulos I. Induction of promoter DNA methylation upon high-pressure spraying of double-stranded RNA in plants. Agronomy. 2021;11:789. doi: 10.3390/agronomy11040789. [DOI] [Google Scholar]
- 116.Mai J., Liao L., Ling R., Guo X., Lin J., Mo B., Chen W., Yu Y. Study on RNAi-based herbicide for Mikania micrantha. Synth. Syst. Biotechnol. 2021;6:437–445. doi: 10.1016/j.synbio.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jiang L., Ding L., He B., Shen J., Xu Z., Yin M., Zhang X. Systemic gene silencing in plants triggered by fluorescent nanoparticle-delivered double stranded RNA. Nanoscale. 2014;6:9965–9969. doi: 10.1039/C4NR03481C. [DOI] [PubMed] [Google Scholar]
- 118.Dubrovina A.S., Aleynova O.A., Kalachev A.V., Suprun A.R., Ogneva Z.V., Kiselev K.V. Induction of transgene suppression in plants via external application of synthetic dsRNA. Int. J. Mol. Sci. 2019;20:1585. doi: 10.3390/ijms20071585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Numata K., Ohtani M., Yoshizumi T., Demura T., Kodama Y. Local gene silencing in plant via synthetic dsRNA and carrier peptide. Plant Biotechnol. J. 2014;12:1027–1034. doi: 10.1111/pbi.12208. [DOI] [PubMed] [Google Scholar]
- 120.Lau S.E., Schwarzacher T., Othman R.Y., Harikrishna J.N. dsRNA silencing of an R2R3-MYB transcription factor affects flower cell shape in a Dendrobium hybrid. BMC Plant Biol. 2015;15:194. doi: 10.1186/s12870-015-0577-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Taning C.N.T., Christiaens O., Berkvens N., Casteels H., Maes M., Smagghe G. Oral RNAi to control Drosophila suzukii: Laboratory testing against larval and adult stages. J. Pest Sci. 2016;89:803–814. doi: 10.1007/s10340-016-0736-9. [DOI] [Google Scholar]
- 122.Kalischuk M.L., Johnson D., Kawchuk L.M. Priming with a double-stranded DNA virus alters Brassicae rapa seed architecture and facilitates a defense response. Gene. 2015;557:130–137. doi: 10.1016/j.gene.2014.12.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.