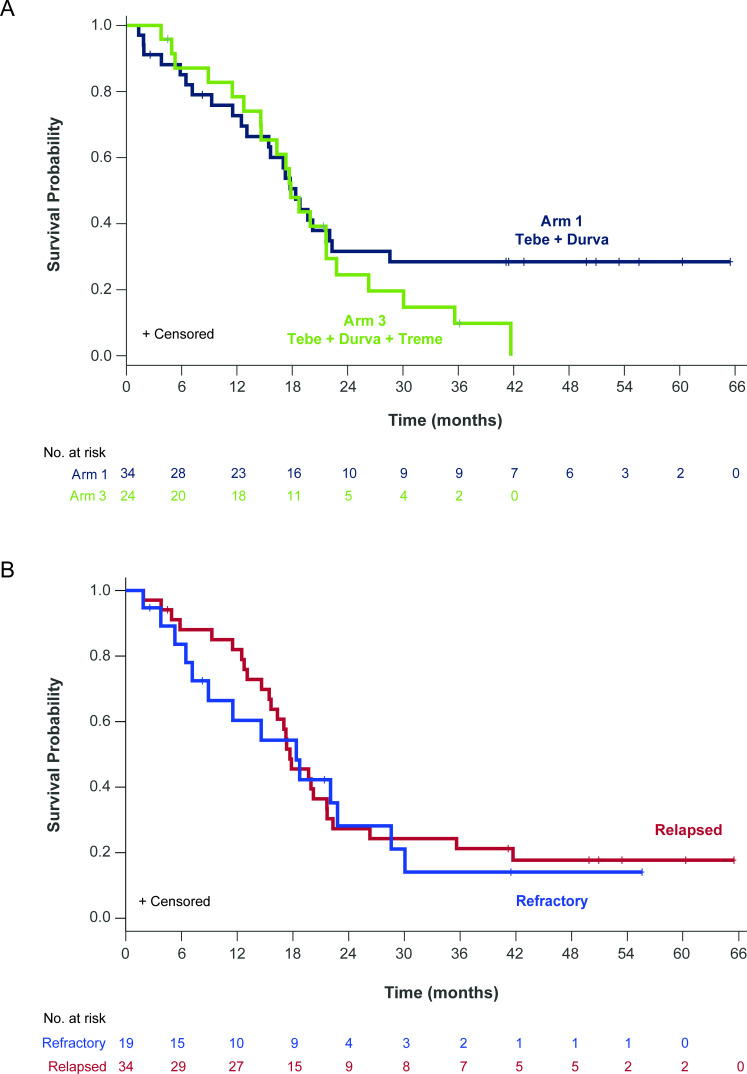
Figure 4.
Overall survival in patients who progressed on prior anti-PD(L)1. (A) Kaplan-Meier estimates of overall survival (OS) for patients who progressed on prior anti-PD(L)1 therapy treated with tebentafusp in combination with durvalumab (Arm 1; n=34; blue) or durvalumab and tremelimumab (Arm 3; n=24; green). Events are deaths due to any cause. Patients not known to have died at the time of analysis are censored. For patients receiving combination tebentafusp and durvalumab (Arm 1), the median OS was 18.4 (95% CI: 13.1 to 22.3) months with a 1-year OS rate of 73% (95% CI: 65% to 80%). For patients receiving triplet therapy (Arm 3), the median OS was 17.8 (95% CI: 14.6 to 21.7) months with a 1-year OS rate of 78% (95% CI: 70% to 87%). (B) Kaplan-Meier estimates of OS for patients who had a best response of progressive disease while on prior anti-PD(L)1 (refractory; n=19; blue) or had a best response of either stable disease (n=17) or partial response/complete response (n=17) and then had disease progression on prior anti-PD(L)1 (relapsed n=34; red). For patients refractory to prior anti-PD(L)1, the median OS was 18.4 (95% CI: 7.2 to 28.6) months with a 1-year OS rate of 60% (95% CI: 49% to 72%). For patients relapsed on anti-PD(L)1, the median OS was 17.7 (95% CI: 15.4 to 21.7) months with a 1-year OS rate of 82% (95% CI: 75% to 89%).