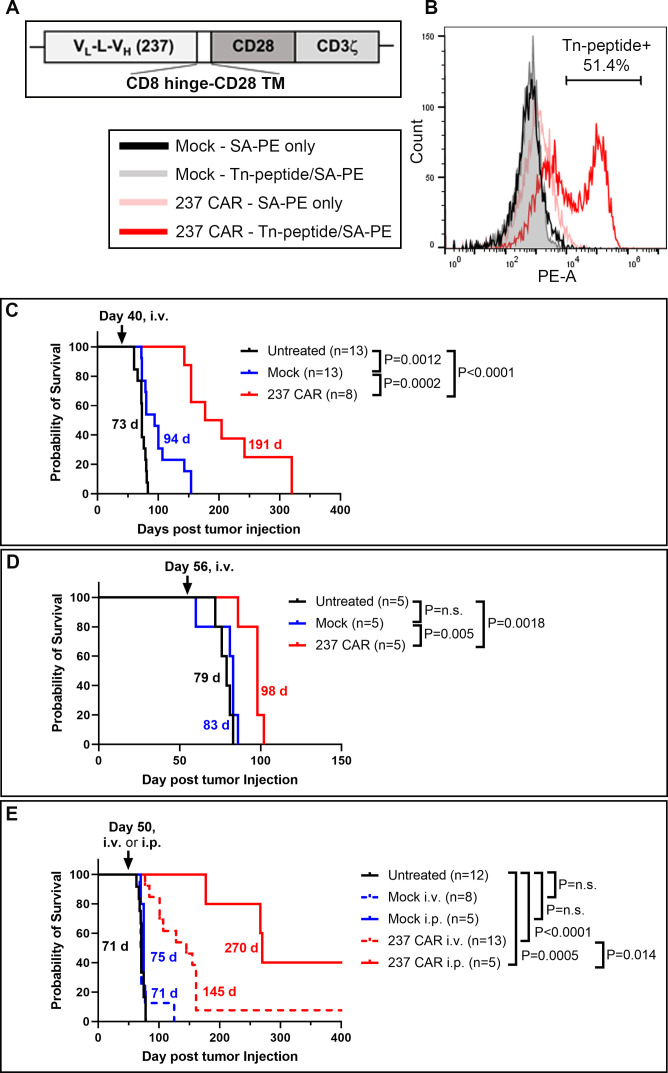
Figure 3.
CAR-T cell treatment of mice with established ID8Cosmc-KO tumors. (A) Schematic of 237 single-chain Fv linked by a CD8 hinge-CD28 transmembrane domain to the CD28 and CD3ζ cytoplasmic domains, cloned into the pMP71 retroviral vector. (B) Primary T cells isolated from splenocytes of naïve donor C57BL/6J mice were transduced with 237 CAR for 72 hours. Transduction efficiency was measured by flow cytometry using tetramers of biotinylated/Tn-glycosylated OTS8 peptide made with streptavidin-PE. Mock-transduced T cells were included as negative control. (C, D) Survival curves of ID8Cosmc-KO-bearing C57BL/6J mice either untreated (black), treated with mock-transduced T cells (blue) or 237 CAR-T cells (red) administered by retro-orbital intravenous injection of 5×106 T cells at day 40 (C) or day 56 (D) post-tumor inoculation. Note that five of the mice in the 237 CAR group (C) were used at day 160 for a re-challenge experiment (figure 7B), such that their survival times may have been even longer without this re-challenge. (E) Comparison of intravenous (dashed red line) and intraperitoneal (solid red line) delivery of 237 CAR-T cells at day 50 post-tumor inoculation. All survival curves were plotted with GraphPad Prism V.9.4.1 using cumulative data across multiple experiments (two combined experiments for C and D, four combined experiments for E), with the indicated total n for each treatment arm. Median survival times are indicated; p values were calculated using the log-rank (Mantel-Cox) test. CAR, chimeric antigen receptor; i.p., intraperitoneal; i.v., intravenous; KO, knock-out.