Abstract
Stress-induced depression and anxiety (DA) are closely connected to gastrointestinal inflammation and dysbiosis, which can suppress brain-derived neurotrophic factor (BDNF) in the brain. Herein, we isolated the BDNF expression-inducing probiotics Lactobacillus casei HY2782 and Bifidobacterium lactis HY8002 in lipopolysaccharide-stimulated SH-SY5Y cells. Then, we investigated the effects of HY2782, HY8002, anti-inflammatory L-theanine, and their supplement (PfS, probiotics-fermented L-theanine-containing supplement) on DA in mice exposed to restraint stress (RS) or the fecal microbiota of patients with inflammatory bowel disease and depression (FMd). Oral administration of HY2782, HY8002, or L-theanine alleviated RS-induced DA-like behaviors. They also decreased RS-induced hippocampal interleukin (IL)-1β and IL-6 levels, as well as NF-κB-positive cell numbers, blood corticosterone level, and colonic IL-1β and IL-6 levels and NF-κB-positive cell numbers. L-theanine more potently suppressed DA-like behaviors and inflammation-related marker levels than probiotics. However, these probiotics more potently increased RS-suppressed hippocampal BDNF level and BDNF+NeuN+ cell numbers than L-theanine. Furthermore, HY2782 and HY8002 suppressed RS-increased Proteobacteria and Verrucomicrobia populations in gut microbiota. In particular, they increased Lachnospiraceae and Lactobacillacease populations, which are closely positively associated with hippocampal BDNF expression, and suppressed Sutterellaceae, Helicobacteriaceae, Akkermansiaceae, and Enterobacteriaceae populations, which are closely positively associated with hippocampal IL-1β expression. HY2782 and HY8002 potently alleviated FMd-induced DA-like behaviors and increased FMd-suppressed BDNF, serotonin levels, and BDNF-positive neuronal cell numbers in the brain. They alleviated blood corticosterone level and colonic IL-1β α and IL-6 levels. However, L-theanine weakly, but not significantly, alleviated FMd-induced DA-like behaviors and gut inflammation. BDNF expression-inducing probiotic (HY2782, HY8002, Streptococcus thermophilus, and Lactobacillus acidophilus)-fermented and anti-inflammatory L-theanine-containing supplement PfS alleviated DA-like behaviors, inflammation-related biomarker levels, and gut dysbiosis more than probiotics or L-theanine. Based on these findings, a combination of BDNF expression-inducing probiotics with anti-inflammatory L-theanine may additively or synergistically alleviate DA and gut dysbiosis by regulating gut microbiota-mediated inflammation and BDNF expression, thereby being beneficial for DA.
Keywords: Lactobacillus casei, restraint stress, depression, anxiety, fecal microbiota
1. Introduction
Stress causes endocrine disruption, immune imbalance, including inflammation, and gut dysbiosis [1,2]. Stress-induced gut dysbiosis is associated with the overproduction of endotoxins such as gram-negative bacterial lipopolysaccharide (LPS), which can cause gut inflammation [3,4,5]. These endotoxins can be translocated to the brain through the blood, and suppress serotonin and brain-derived neurotropic factor (BDNF) levels in the hippocampus, resulting in psychiatric disorders including depression and anxiety (DA) through neuroinflammation [4,6,7].
Probiotics improve gut dysbiosis, metabolic diseases, immune imbalance, and neurodegenerative disorders [8,9,10,11]. Bifidobacterium longum NCC3001 alleviates depression and colitis in rodents [12]. Moreover, NCC3001 partially alleviates depression scores in patients with irritable bowel syndrome [13]. NVP1704, a Lactobacillus reuteri and Bifidobacterium adolescentis mix, improves restraint stress (RS)-related DA-like behaviors and sleep disturbances in volunteers with depression and insomnia [14]. NVP1704 also alleviates Escherichia coli-induced DA in mice [15]. Bifidobacterium breve CCFM1025 mitigates depression in rodents treated with chronic unpredictable mild stress (CUMS) by alleviating gut microbial abnormalities [16]. Bifidobacterium breve CCFM1025 reduces depressive scores in volunteers with major depression disorder by modulating gut microbiota and serotonin turnover [17]. Lactobacillus rhamnosus HN001 improves DA in CUMS-treated mice and pregnancy [18,19]. Lactobacillus pentosus NK357 also mitigates gut inflammation (GI) and gut dysbiosis (GD) in pathogen-exposed mice [20].
Some herbs, such as lavender, passionflower, saffron, and green tea have a well-known ability to alleviate DA [21,22]. Green tea contains polyphenols (especially epigallocatechin-3-gallate), polysaccharides, L-theanine, and caffeine [23]. In particular, L-theanine mitigates CUMS-induced depression in rats and DA-related symptoms in healthy volunteers [24]. However, the effects of supplements combined with probiotics and herbal constituents for the treatment of stress-induced DA have been not sufficiently studied.
Therefore, to understand the combined effects of BDNF expression-inducing probiotics and anti-inflammatory natural product constituent L-theanine on DA, we first isolated BNDF expression-inducing probiotics in LPS-stimulated SH-SY5Y cells, then investigated the effects of BDNF expression-inducing probiotics (Lactobacillus casei HY2782 and Bifidobacterium lactis HY8002), anti-inflammatory L-theanine, and their supplement on DA in RS-exposed or gut microbiota-transplanted mice.
2. Materials and Methods
2.1. Bacterial Strains
HY2782 and HY8002 were isolated from human fecal lactic acid bacteria collection, and Streptococcus thermophilus and Lactobacillus acidophilus strains were purchased from CSI (Culture System Inc., Mishawaka, IN, USA) as commercial strains for starter culture. Probiotics were cultured in MRS broth (BD, Franklin Lakes, NJ, USA) at 37 °C for 18 h, centrifuged (4000× g, 4 °C, 10 min), and washed with saline twice. The collected cells were resuspended in saline for in vivo experiments and heat (75 °C for 15 min)-tyndallized for in vitro experiments.
2.2. Preparation of Probiotics-Fermented Supplement (pFS)
For the preparation of PfS, L. casei (HY2782), Bifidobacterium lactis (HY8002), Streptococcus thermophilus, and Lactobacillus acidophilus, which were contained in the PROBIOTICS ABCT-3 starter culture (Culture System Inc., Mishawaka, IN, USA), were fermented in fresh milk, skim milk powder, dairy cream, and lactase (<0.002%, Maxilact LGi 5000, DSM Food Specialities, Libercourt, France); L-theanine (>98% pure synthetic L-theanine, Hunan Nutramax Inc., Hunan, Changsha, China) was added after the fermentation. In the final supplement, L-theanine content was 2 mg/mL, and total probiotic population was 2 × 108 colony forming units (CFU)/mL.
2.3. SH-SY5Y Cell Culture and Selection of BDNF Expression-Inducing Probiotics
SH-SY5Y cells were cultured in DMEM containing 1% antibiotic-antimycotic and 5% FBS (37 °C, 95% air/5% CO2). For the BDNF expression assay, SH-SY5Y cells (1 × 106 cells/mL) were incubated with LPS (100 ng/mL) in the absence or presence of probiotics (1 × 104 or 1 × 106 CFU/mL) for 24 h. BDNF levels were assayed using a commercial enzyme-linked immunosorbent assay (ELISA) kit.
2.4. Animals
C57BL/6 mice (male, 7 weeks old) were provided from Samtaco Inc. (Osan-shi, Seoul, Republic of Korea), maintained in a controlled room with water and food ad libitum, and acclimatized for 7 days before the use of experiment. All animal experiments were approved by the Committee for the Care and Use of Laboratory Animals in Kyung Hee University (IACUC No, KHUASP(SE)-23005) and were ethically carried out in accordance with the Guidelines of the University for Laboratory Animals Care and Use.
Mice with DA were prepared as previously reported [25]. (1) For the preparation of mice with RS-induced DA, mice were exposed to RS daily for 7 days. From the next day, test agents (Hy1, 2 × 108 CFU/mouse of HY2782; Hy2, 2 × 108 CFU/mouse of HY8002; Tn, 2 mg/kg of L-theanine; PfS, fermented supplement containing 2 × 108 CFU/mouse of probiotics (consisted of L. casei, B. lactis, S. thermophilus, and L. acidophilus) and 2 mg/kg of L-theanine) were orally administered daily for 10 days. (2) For the preparation of mice with DA induced by fecal microbiota of patients with inflammatory bowel disease and depression (FMd), first FMd was cultured in general anaerobic medium (GAM) broth (Nissui Pharm. Co., Tokyo, Japan), as previously reported [25], centrifuged, and washed with saline twice. The collected FMd was orally transplanted into mice daily for 5 days. From the next day, test agents (Hy1, 2 × 108 CFU/mouse of HY2782; Hy2, 2 × 108 CFU/mouse of HY8002; Tn, 2.0 mg/kg of L-theanine; PfS, fermented supplement containing 2 × 108 CFU/mouse of probiotics (consisted of L. casei, B. lactis, S. thermophilus, and L. acidophilus) and 2 mg/kg of L-theanine) were then orally administered daily for 10 days.
DA-like behaviors (one task in one day) were assessed from the next day after the final probiotic treatment. Mice were euthanized by exposure to CO2 in a chamber and then sacrificed by cervical dislocation. Sera, brains, colons, and feces were collected and stored at −80 °C for biomarker assays.
2.5. Behavioral Tasks
The open field test (OFT) was performed in a chamber (40 × 40 cm; center zone, 20 × 20 cm) equipped with a record camera for 10 min and quantified using the EthoVision XT software [26]. The elevated plus maze task (EPMT) and tail suspension test (TST) were assessed in the plus-maze apparatus and table edge, as previously reported [25].
2.6. Eenzyme-Linked Immunosorbent Assay (ELISA)
Hippocampus, hypothalamus, and colon tissues were lysed in a RIPA lysis buffer and centrifuged, as previously reported [4]. Sera were prepared as previously reported [20]. BDNF, IL-1β, IL-2, IL-6, and tumor necrosis factor (TNF)-α levels were determined using commercial ELISA kits (R&D Systems, Minneapolis, MN, USA) [27].
2.7. Immunofluorescence Staining
Mice were transcardially perfused with paraformaldehyde. Their hypothalamus and colon tissues were sectioned and incubated with primary antibodies for 12 h, then treated with secondary antibodies and observed using a confocal microscope, as previously reported [28]. The sections were incubated with primary antibodies against BDNF, NeuN, NF-κB, Iba1, and/or CD11c for 12 h, then treated with secondary antibodies conjugated with Alexa Fluor 594 or Alexa Fluor 488 (1:200, Invitrogen, Waltham, MA, USA) for 2 h, and observed using a confocal microscope.
2.8. Microbiota Analysis
Microbiota genomic DNA was extracted from the stool of mice using a QIAamp DNA stool mini kit. Next, 16S rRNA genes were amplified and sequenced, as previously reported [29]. Sequenced data were deposited in NCBI (PRJNA962265).
2.9. Statistics
Data are expressed as mean ± S.D. using GraphPad Prism 9. The significant differences were analyzed using one-way ANOVA followed by Duncan’s multiple range test (p < 0.05). The correlation between gut microbiota and BDNF expression or IL-1β level was analyzed using the Spearman correlation coefficient.
3. Results
3.1. L. casei and B. lactis Up-Regulated LPS-Suppressed BDNF Expression in SH-SY5Y Cells
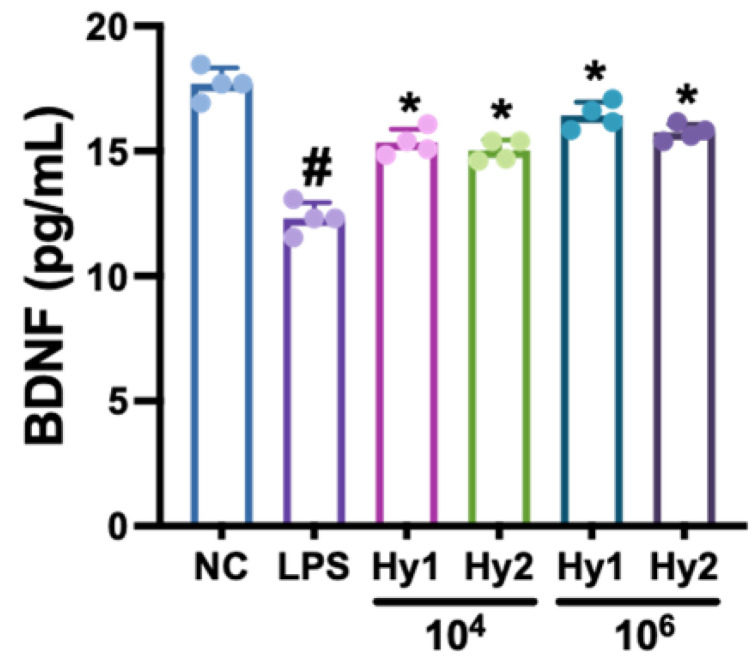
To select stress-induced DA-ameliorating probiotics, we screened BDNF expression-inducing probiotics from our lactic acid bacteria collection. Of the tested bacteria, L. casei HY2782 and B. lactis HY8002 potently increased LPS-suppressed BDNF expression in SH-SY5Y cells compared to that of the normal control (NC) (Figure 1).
Figure 1.
Effects of L. casei HY2782 and B. lactis HY8002 on LPS-suppressed BDNF expression in SH-SY5Y cells. Probiotics (Hy1, HY2782; Hy2, HY8002) were administered at a dose of 1 × 104 or 1 × 106 CFU/mL. LPS and NC mice were treated with LPS (100 ng/mL) or saline, respectively. Dn = 4. # p < 0.05 vs. NC. * p < 0.05 vs. LPS.
3.2. L. casei, B. lactis, L-Theanine, and Their Supplement PfS Improved RS-Induced DA-like Behaviors and Neuroinflammation in Mice
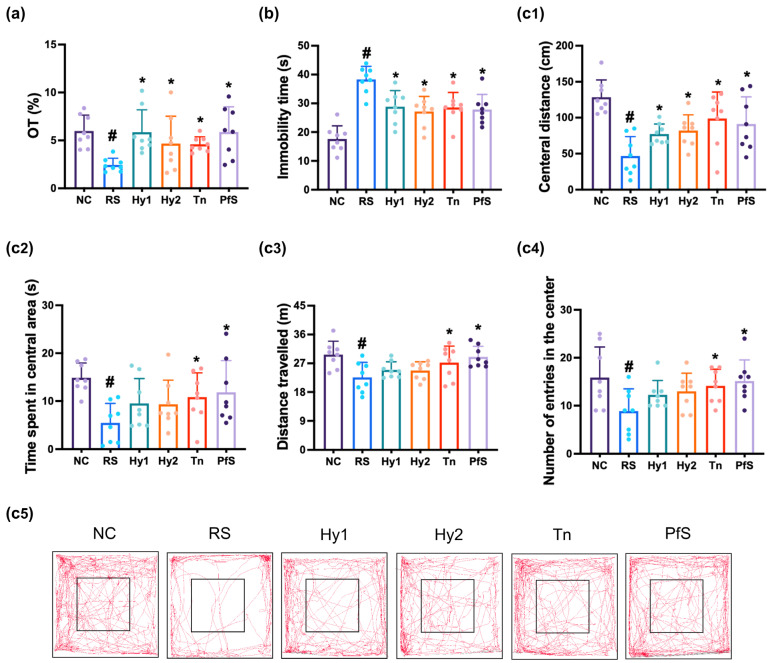
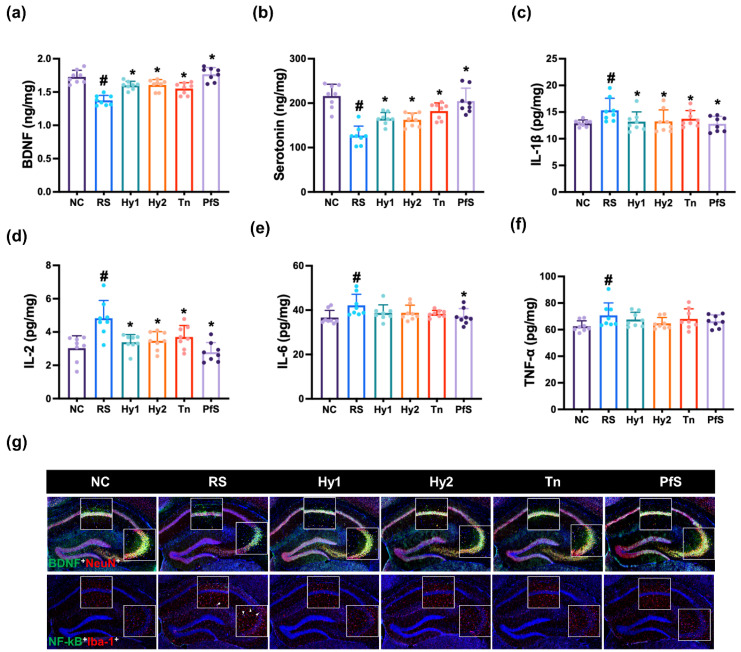
To study whether BDNF level-increasing probiotics could ameliorate DA in vivo, we investigated the effects of HY2782, HY8002, L-theanine, and their supplement PfS on RS-induced DA-like behaviors in mice (Figure 2). In NC mice, exposure to RS significantly decreased time in the open arm (OT) during the EPMT to 40.9% (F(6, 49) = 3.015, p < 0.014), reduced the central distance (CD), time spent in central area (CT), distance travelled (TD), and number of entries in the center (CN) during the OFT to 36.3% (F(6, 49) = 6.670, p < 0.001), 36.7% (F(6,4 9) = 2.925, p < 0.016), 76.1% (F(6, 49) = 3.478, p < 0.006), and 55.9% (F(6, 49) = 2.534, p < 0.032]), respectively, and expanded immobility time in the TST to 216.9% (F(6, 49) = 10.320, p < 0.001). However, HY2782, HY8002, and L-theanine increased RS-suppressed OT to 98.0%, 78.3%, and 76.9% (F(6, 49) = 3.015, p < 0.014), respectively, in NC mice. They also suppressed RS-increased immobility time to 163.6%, 154.1%, and 162.8% (F(6, 49) = 10.320, p < 0.001), respectively, and increased RS-suppressed CD to 60.1%, 63.9%, and 76.8% (F(6, 49) = 2.534, p < 0.032), respectively, in NC mice. Although L-theanine also potently alleviated RS-suppressed CT, TD, and CN, HY2782 and HY8002 weakly, but not significantly, increased them. They significantly decreased IL-1β and IL-2 levels in the hippocampus, while TNF-α levels were not affected (Figure 3). Although L-theanine significantly suppressed IL-6 expression, these probiotics did not significantly suppress it. They increased RS-suppressed serotonin, BDNF levels, and BDNF+NeuN+ cell population.
Figure 2.
Effects of L. casei HY2782, B. lactis HY8002, L-theanine, and their supplement on RS-induced DA-like behaviors in mice. Behaviors were assessed in the EPMT (a), TST (b), and OPT (c: (c1), CD; (c2), CT; (c3), TN; (c4), CN; (c5), track path). Test agents (RS, saline; Hy1, HY2782; Hy2, HY8002; Tn, L-theanine; PfS, probiotics-fermented L-theanine-containing supplement) were orally gavaged. NC mice were treated with vehicle. n = 8. # p < 0.05 vs. NC. * p < 0.05 vs. RS.
Figure 3.
Effects of L. casei HY2782, B. lactis HY8002, L-theanine, and their supplement on RS-induced neuroinflammation in mice. Effects on hippocampal BDNF (a), serotonin (b), IL-1β (c), IL-2 (d), IL-6 (e), and TNF-α levels (f), and NF-κB+Iba1+ and BDNF+NeuN+ cell numbers (g). Test agents (RS, saline; Hy1, HY2782; Hy2, HY8002; Tn, L-theanine; PfS, probiotics-fermented L-theanine-containing supplement) were orally gavaged. NC mice were treated with vehicle. n = 8. # p < 0.05 vs. NC. * p < 0.05 vs. RS.
Treatment with PfS alleviated RS-induced DA-like behaviors in mice (Figure 2). PfS increased RS-suppressed OT to 98.5% (F(6, 49) = 3.015, p < 0.014), CD to 71.0% (F(6, 49) = 6.670, p < 0.001), and CT to 79.4% (F(6, 49) = 2.925, p < 0.016), in NC mice. PfS suppressed RS-exposed immobility time to 157.9% (F(6, 49) = 2.534, p < 0.032) in NC mice. Furthermore, PfS increased RS-suppressed serotonin, BDNF levels, and BDNF+NeuN+ cell numbers in the hippocampus, while RS-induced IL-1β and IL-6 levels and NF-κB+Iba1+ cell numbers decreased (Figure 3). PfS alleviated DA-like behaviors and neuroinflammation more potently than probiotics or L-theanine alone.
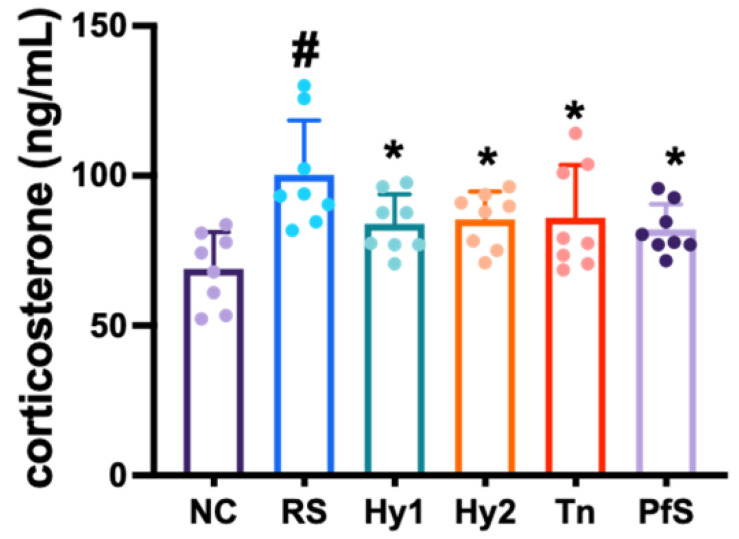
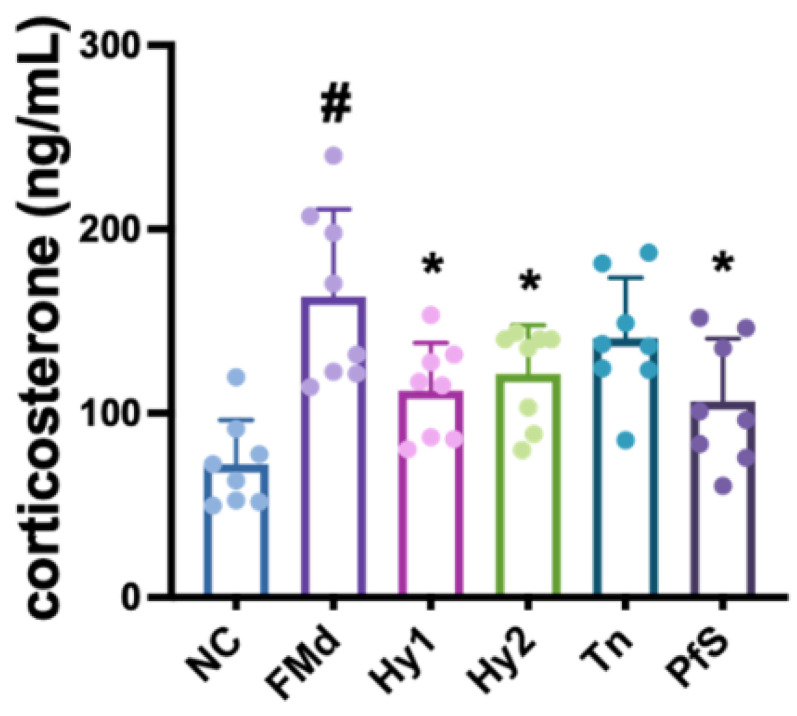
Exposure to RS increased corticosterone levels in the blood (Figure 4). However, HY2782, HY8002, L-theanine, and PfS lowered RS-induced corticosterone levels.
Figure 4.
Effects of L. casei HY2782, B. lactis HY8002, L-theanine, and their supplement on corticosterone level in RS-exposed mice. Test agents (RS, saline; Hy1, HY2782; Hy2, HY8002; Tn, L-theanine; PfS, probiotics-fermented L-theanine-containing supplement) were orally gavaged. NC mice were treated with vehicle. n = 8. # p < 0.05 vs. NC. * p < 0.05 vs. RS.
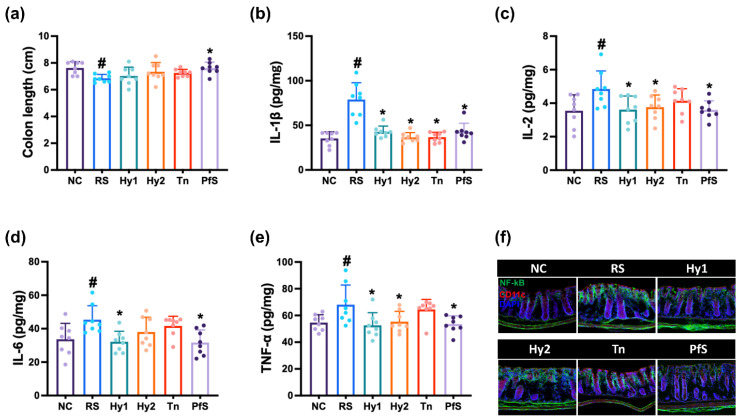
3.3. L. casei, B. lactis, L-Theanine, and Their Supplement Improved RS-Induced GI and GD in Mice
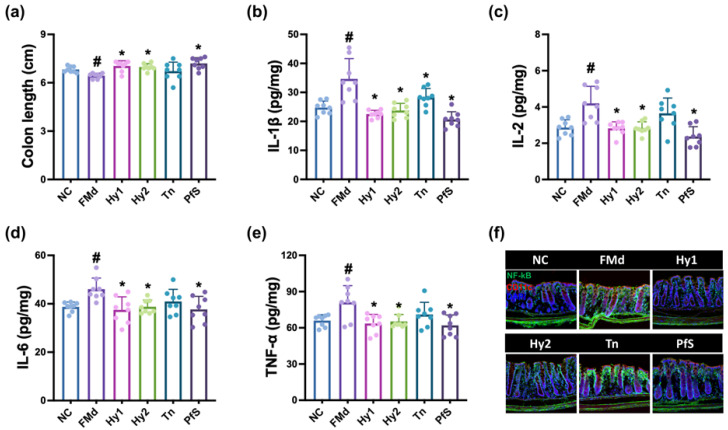
The effects of HY2782 and HY8002 on RS-induced GI were investigated in the colon of mice (Figure 5). Exposure to RS induced colon shortening and increased IL-1β, IL-2, IL-6, and TNF-α levels, as well as NF-κB-positive cell numbers. However, HY2782 and HY8002 decreased RS-increased IL-1β and IL-6 levels, and NF-κB-positive cell numbers. L-theanine did not affect IL-1β, IL-2, IL-6, or TNF-α levels. PfS strongly suppressed IL-1β, IL-6, and TNF-α levels, as well as NF-κB-positive cell numbers, compared to those of L-theanine or probiotics.
Figure 5.
Effects of L. casei HY2782, B. lactis HY8002, L-theanine, and their supplement on RS-induced GI in mice. Effects on colon length (a), IL-1β (b), IL-2 (c), IL-6 (d), and TNF-α (e) levels, and NF-κB+CD11c+ cell numbers (f). Test agents (RS, saline; Hy1, HY2782; Hy2, HY8002; Tn, L-theanine; PfS, probiotics-fermented L-theanine-containing supplement) were orally administered. NC mice were treated with saline. n = 8. # p < 0.05 vs. NC. * p < 0.05 vs. RS.
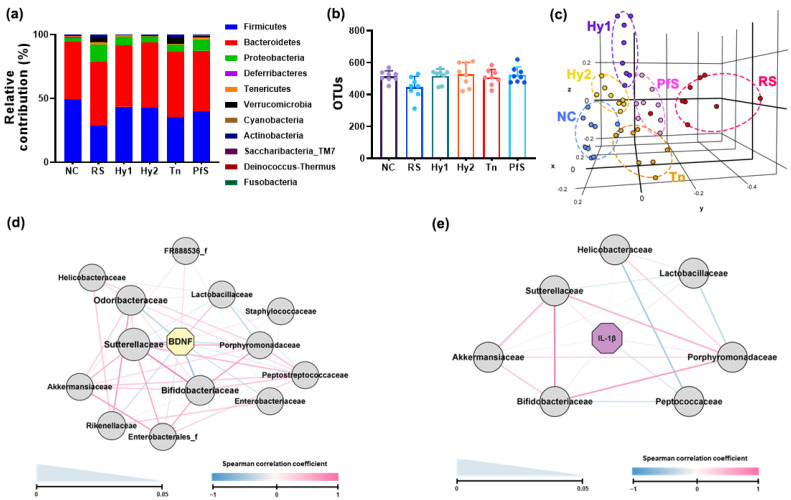
The effects of probiotics, L-theanine, and their supplement on RS-induced gut microbiota alteration were investigated in mice (Figure 6, Supplementary Materials, Tables S1–S3). Exposure to RS-altered fecal microbiota composition: Exposure weakly, but not significantly, decreased α-diversity (OTUs) and shifted β-diversity (PCoA). RS increased Proteobacteria, Verrucomicrobia, and Actinobacteria populations, and decreased Firmicutes and Deferribacteres populations. However, HY2783, HY8002, and PfS suppressed RS-increased Proteobacteria and Verrucomicrobia populations and increased Firmicutes and Deferribacteres populations. Although L-theanine weakly, but not significantly, increased RS-suppressed Firmicutes population, it did not significantly affect RS-increased Verrucomicrobia or Actinobacteria populations. Of the gut microbiota, RS-decreased Ruminococcaceae and Lactobacillacease populations showed a positive correlation with BDNF expression levels, while RS-increased Sutterellaceae, Helicobacteriaceae, Akkermansiaceae, Enterobacteriaceae, and Bifidobacteriaceae numbers had a negative correlation. IL-1β expression levels showed a positive correlation with Sutterellaceae, Bifidobacteiralceae, and Helicobacteriaceae populations, while Lactobacillaceae and Peptococcaceae populations had a negative correlation.
Figure 6.
Effects of L. casei HY2782, B. lactis HY8002, L-theanine, and their supplement on RS-induced fecal microbiota alteration in mice. Effects on gut microbiota composition: (a), phylum level; (b), OTUs (α-diversity); (c), β-diversity (PCoA plot based on Jensen-Shannon analysis). The interrelation between gut microbiota and BDNF ((d), Spearman correlation coefficient) or IL-1β expression level ((e), spearman correlation coefficient). Test agents (RS, saline; Hy1, HY2782; Hy2, HY8002; Tn, L-theanine; PfS, probiotics-fermented L-theanine-containing supplement) were orally gavaged. n = 8.
3.4. L. casei, B. lactis, and Its Supplement Improved FMd-Induced DA-like Behaviors and Neuroinflammation in Mice
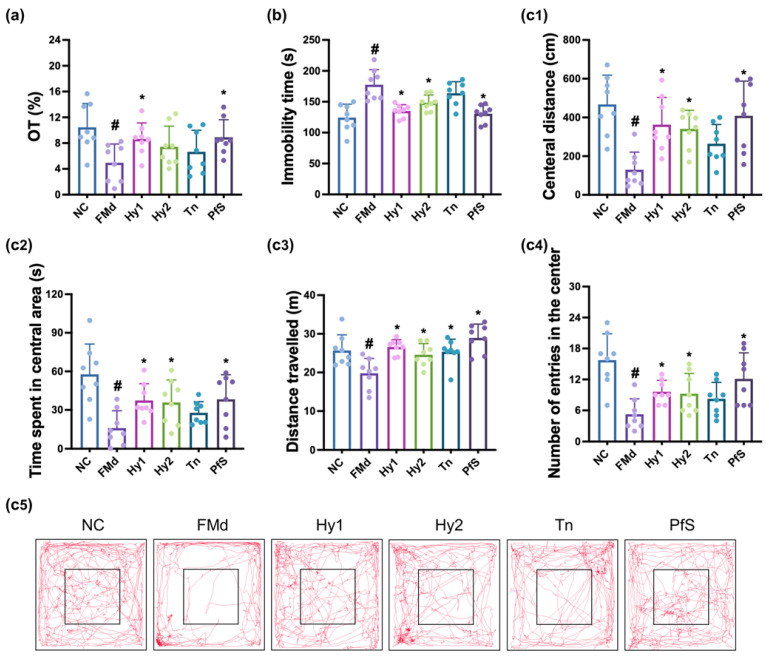
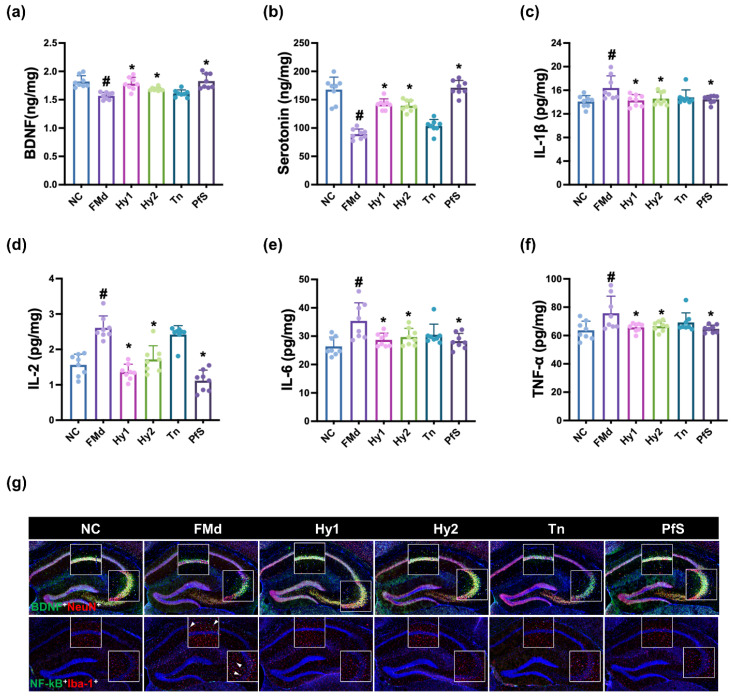
FMd transplantation causes DA with GI in mice [25]. To confirm the anti-depressive effects of HY2782, HY8002, and PfS, we examined their effects on FMd-induced DA in mice (Figure 7). In NC mice, FMd transplantation significantly decreased OT to 50.9% (F(5, 42) = 3.116, p < 0.018), reduced CD, CT, TD, and CN to 27.8% (F(5, 42) = 6.637, p < 0.001), 27.6% (F(5, 42) = 5.494, p < 0.001), 77.0% (F(5, 42) = 6.598, p < 0.001), and 33.3% (F(5, 42) = 6.756, p < 0.001), respectively, and increased immobility time to 142.8% (F(5, 42) = 11.000, p < 0.001). However, oral administration of HY2782 and HY8002 increased FMd-suppressed OT to 88.8% and 76.4% (F(5, 42) = 3.116, p < 0.018), respectively, in NC mice. They also decreased FMd-increased immobility time to 108.6% and 118.2% (F(5, 42) = 11.000, p < 0.001), respectively, and increased FMd-decreased CD to 78.0% and 72.9% (F(5, 42) = 6.637, p < 0.001), respectively, and CT to 64.7% and 62.1% (F(5, 42) = 5.494, p < 0.001), repectively, in NC mice. They significantly suppressed FMd-induced hippocampal TNF-α, IL-1β, IL-2, and IL-6 expression, while FMd-suppressed BDNF levels and BDNF+NeuN+ cell numbers increased (Figure 8). However, L-theanine did not significantly alleviate the DA-like behaviors of OT, immobility time, CD, or CT. Moreover, L-theanine weakly, but not significantly, suppressed FMd-induced proinflammatory cytokine expression in the hippocampus.
Figure 7.
Effects of L. casei HY2782, B. lactis HY8002, L-theanine, and their supplement on FMd-induced DA-like behaviors in mice. Behaviors were assessed in the EPMT (a), TST (b), and OPT (c: (c1), CD; (c2), CT; (c3), TN; (c4), CN); (c5), track path). Test agents (FMd, saline; Hy1, HY2782; Hy2, HY8002; Tn, L-theanine; PfS, probiotics-fermented L-theanine-containing supplement) were orally gavaged. NC mice were treated with vehicle (saline) instead of test agents. n = 8. # p < 0.05 vs. NC. * p < 0.05 vs. FMd.
Figure 8.
Effects of L. casei HY2782, B. lactis HY8002, L-theanine, and their supplement on FMd-induced neuroinflammation in mice. Effects on hippocampal BDNF (a), serotonin (b), IL-1β (c), IL-2 (d), IL-6 (e), and TNF-α levels (f), and NF-κB+Iba1+ and BDNF+NeuN+ cell numbers (g). Test agents (FMd, saline; Hy1, HY2782; Hy2, HY8002; Tn, L-theanine; PfS, probiotics-fermented L-theanine-containing supplement) were orally gavaged. NC mice were treated with vehicle (saline). n = 8. # p < 0.05 vs. NC. * p < 0.05 vs. FMd.
Treatment with PfS alleviated FMd-induced DA-like behaviors in mice. PfS increased FMd-suppressed OT to 86.5% (F(5, 42) = 3.116, p < 0.018), CD to 87.4% (F(5, 42) = 6.637, p < 0.001), and CT to 66.3% (F(5, 42) = 5.494, p < 0.001) in NC mice. Furthermore, PfS decreased FMd-increased immobility time to 105.0% (F(5, 42) = 11.000, p < 0.001) in NC mice. PfS also increased FMd-suppressed hippocampal serotonin, BDNF levels, and BDNF+NeuN+ cell numbers, while FMd-induced hippocampal TNF-α, IL-1β, and IL-6 levels, as well as NF-κB+Iba1+ cell numbers, decreased. PfS alleviated depression-like behaviors and neuroinflammation more potently than probiotics or L-theanine alone.
FMd transplantation increased corticosterone levels in the blood (Figure 9). However, HY2782, HY8002, L-theanine, and PfS all lowered FMd-induced corticosterone levels.
Figure 9.
Effects of L. casei HY2782, B. lactis HY8002, L-theanine, and their supplement on corticosterone level in RS-exposed mice. Test agents (FMd, saline; Hy1, HY2782; Hy2, HY8002; Tn, L-theanine; PfS, probiotics-fermented L-theanine-containing supplement) were orally gavaged. NC mice were treated with vehicle. n = 8. # p < 0.05 vs. NC. * p < 0.05 vs. FMd.
3.5. L. casei, B. lactis, L-Theanine, and Their Supplement Improved FMd Transplantation-Induced GI in Mice
The effects of HY2782 and HY8002 on FMd-induced GI were investigated in the colon of mice (Figure 10). FMd transplantation increased IL-1β, IL-2, IL-6, and TNF-α levels, as well as NF-κB-positive cell numbers. However, HY2782 and HY8002 decreased IL-1β, IL-6, and TNF-α levels, as well as NF-κB-positive cell populations. However, L-theanine weakly suppressed these compared to probiotics. Treatment with PfS strongly suppressed the above levels compared to L-theanine or probiotics alone.
Figure 10.
Effects of L. casei HY2782, B. lactis HY8002, L-theanine, and their supplement on FMd-induced GI in the colon of mice. Effects on colon length (a), IL-1β (b), IL-2 (c), IL-6 (d), and TNF-α (e) levels, and NF-κB+CD11c+ cell number (f). Test agents (FMd, saline; Hy1, HY2782; Hy2, HY8002; Tn, L-theanine; PfS, probiotics-fermented L-theanine-containing supplement) were orally gavaged. Normal control (NC) was treated with saline. n = 8. # p < 0.05 vs. NC. * p < 0.05 vs. FMd.
4. Discussion
Exposure to stressors induces the secretion of adrenocorticotropic hormone from the pituitary gland, which the stimulates the excretion of cortisol (corticosterone) from the adrenal gland through the hypothalamic–pituitary–adrenal axis and activates NF-κB signaling [30,31], while serotonin and BDNF levels decrease in the central nervous system and gastrointestinal tract, resulting in DA with neuroinflammation and colitis [32,33]. In the present study, exposure to RS increased blood corticosterone and IL-6 levels, and decreased hippocampal BDNF and serotonin levels, as well as BDNF-positive neuron cells. Moreover, exposure to RS increased IL-1β levels and NF-κB-positive cells in the brain and colon. RS increased DA-like behaviors. Furthermore, RS increased the gut Proteobacteria and Verrucomicrobia populations. Hippocampal BDNF expression levels were positively correlated with Ruminococcaceae and Lactobacillacease populations, which were negatively correlated with the expression levels of inflammatory cytokines such as IL-1β. Hippocampal BDNF expression levels were negatively correlated with Sutterellaceae, Helicobacteri-aceae, Akkermansiaceae, and Enterobacteriaceae populations, which were positively correlated with the expression levels of inflammatory cytokines such as IL-1β. Peirce and Alvina suggested that RS-induced depression could cause GI and GD [34]. Jang et al. reported that RS increased blood and fecal LPS levels through GD [3]. These observations suggest that RS may cause GI and DA by suppressing corticosterone-mediated BDNF and serotonin expression, and inducing corticosterone-mediated NF-κB signaling through LPS-overexpressed GD.
Here, we selected BDNF expression-increasing probiotics L casei HY2782 and B. lactis HY8002 in LPS-treated SH-SY5Y cells. These probiotics increased RS-suppressed BDNF and serotonin levels in the brain. However, they weakly suppressed RS-induced hippocampal and colonic proinflammatory cytokine and NF-κB-positive cell levels. Moreover, they weakly reduced blood corticosterone levels, and weakly alleviated RS-induced DA-like behaviors. Nevertheless, they shifted RS-fluctuated gut microbiota composition to that of NC. In particular, these probiotics suppressed RS-increased Proteobacteria and Verrucomicrobia populations. Furthermore, they increased Lachnospiraceae and Lactobacillacease populations, which are closely positively associated with hippocampal BDNF expression, and suppressed Sutterellaceae, Helicobacteriaceae, Akkermansiaceae, and Enterobacteriaceae populations, which are closely positively associated with hippocampal IL-1β expression. L-theanine also increased serotonin and BDNF levels, similarly to HY2782. However, L-theanine showed an anti-depressive effect. These results suggest that L-theanine may express its anti-depressive effects by regulating depressive factors such as neuropeptide Y and adrenaline [35], differently from probiotics.
Interestingly, HY2782 and HY8002 potently alleviated FMd-increased DA-like behaviors. They decreased FMd-induced hippocampal and colonic proinflammatory cytokine expression, and increased FMd-suppressed hippocampal BDNF expression and BDNF-positive neuron cell numbers. L-theanine strongly alleviated RS-induced DA-like behaviors associated with neuroinflammation and colitis. Its effects were more potent than those of probiotics in mice with RS-induced DA. However, L-theanine treatment hardly affected RS-fluctuated gut microbiota or hippocampal BDNF level. L-theanine hardly induced LPS-suppressed BDNF expression in vitro (Supplementary Materials, Figure S1). However, L-theanine weakly alleviated FMd-induced DA-like behaviors. L-theanine also weakly decreased the expression of IL-1β, IL-6, and TNF-α, which are neuroinflammation- and colitis-related biomarkers [36,37]. Zhang et al. found that L-theanine suppressed colitis in rodents by suppressing NF-κB signaling [38]. Gut inflammation is closely associated with the outbreak of psychiatric disorders, including DA and gut dysbiosis [4,39,40]. DA causes gut dysbiosis [41], which alters gut microbiota composition and overexpresses microbiota toxins such as LPS, and inflammation [25], which further induces DA [42]. Proinflammatory cytokines suppress BDNF and serotonin production in neuron cells [43]. The suppression of systemic inflammation alleviates DA in vivo [25]. We found that RS and FMd transplantation increased hippocampal and colonic IL-1β and IL-6 levels, as well as NF-κB-positive cell numbers, while hippocampal serotonin and BDNF levels decreased, as previously reported [25]. However, FMd transplantation more potently increased colonic IL-1β and IL-6 levels, as well as blood corticosterone levels, in mice that had been exposed to RS. These observations imply that BDNF expression-inducing probiotics, in particular L. casei, may alleviate RS-induced DA with gut dysbiosis through the modulation of gut microbiota composition and BDNF expression, while L-theanine may alleviate DA with neuroinflammation and colitis through the suppression of NF-κB activation.
Treatment with PfS, which is a probiotics-fermented L-theanine-containing supplement, alleviated RS- or FMd-induced DA-like behaviors, decreased hippocampal IL-1β and IL-6 levels, and increased RS- or FMd-suppressed hippocampal BDNF, serotonin levels, and BDNF-positive neuron cell numbers more potently than probiotics or L-theanine alone. Treatment also suppressed RS- or FMd-increased blood corticosterone levels, colonic IL-1β and IL-6 levels, and NF-κB-positive cell numbers more than probiotics or L-theanine alone. Furthermore, PfS suppressed Proteobacteria and Verrucomicrobia populations in RS-exposed mice. PfS also increased Lactobacillacease populations, which is closely positively associated with hippocampal BDNF expression, and suppressed Sutterellaceae, Helicobacteriaceae, Akkermansiaceae, and Enterobacteriaceae populations, which are closely positively associated with hippocampal IL-1β expression.
These observations suggest that the combination of BDNF expression-inducing probiotics, including HY2782 and HY8002, with anti-inflammatory L-theanine may additively or synergistically alleviate stress-induced DA by modulating gut microbiota-mediated inflammation and BDNF expression.
5. Conclusions
BDNF expression-inducing L. casei HY2782 and B. lactis HY8002 alleviated RD- or fecal microbiota-induced DA-like behaviors, neuroinflammation, and GI with GD by suppressing gut microbiota-mediated NF-κB activation and inducing BDNF expression. L-theanine alleviated RS-induced DA-like behaviors by suppressing NF-κB signaling. The combination of BDNF expression-inducing probiotics, including HY2782 and HY8002, with anti-inflammatory L-theanine may additively alleviate DA by modulating gut microbiota-mediated inflammation and BDNF expression, thereby being beneficial for DA.
Abbreviations
BDNF, brain-derived neurotropic factor; CD, central distance; CN number of entries in the center; CT, central area-spent time; CUMS, chronic unpredictable mild stress; DA, depression and anxiety; ELISA, enzyme-linked immunosorbent assay; EPMT, elevated plus maze task; GAM, general anaerobic medium; LPS, lipopolysaccharide; OFT, open field test; NC, normal control; SD, standard deviation; TD, distance travelled; TST, tail suspension test.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15112488/s1, Table S1. Effects of HY2782 and its supplements on the fecal microbiota composition (at the phylum level). Table S2. Effects of HY2782 and its supplements on the fecal microbiota composition (at the family level). Table S3. Effects of HY2782 and its supplements on the fecal microbiota composition (at the genus level). Figure S1. Effect of L-theanine on LPS-suppressed BDNF expression in SH-SY5Y cells.
Author Contributions
Conceptualization and experiment design, X.M., J.-L.L. and D.-H.K.; experiment and data analysis, X.M., Y.-J.S., H.-S.P., J.-W.J., J.Y.K. and J.-J.S.; investigation, X.M., Y.-J.S. and H.-S.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All animal experiments were approved by the Committee for the Care and Use of Laboratory Animals in Kyung Hee University (IACUC No, KHUASP(SE)-23005) and were ethically carried out in accordance with the Guidelines of the University for Laboratory Animals Care and Use.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data analyzed during the present study are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the MRC Program through NRF funded Ministry of Science and ICT (2017R1A5A2014768).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Madison A., Kiecolt-Glaser J.K. Stress, depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019;28:105–110. doi: 10.1016/j.cobeha.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Misiak B., Łoniewski I., Marlicz W., Frydecka D., Szulc A., Rudzki L., Samochowiec J. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2020;102:109951. doi: 10.1016/j.pnpbp.2020.109951. [DOI] [PubMed] [Google Scholar]
- 3.Jang H.M., Lee K.E., Lee H.J., Kim D.H. Immobilization stress-induced Escherichia coli causes anxiety by inducing NF-κB activation through gut microbiota disturbance. Sci. Rep. 2018;8:13897. doi: 10.1038/s41598-018-31764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J.K., Lee K.E., Lee S.A., Jang H.M., Kim D.H. Interplay Between Human Gut Bacteria Escherichia coli and Lactobacillus mucosae in the Occurrence of Neuropsychiatric Disorders in Mice. Front. Immunol. 2020;11:273. doi: 10.3389/fimmu.2020.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim K.A., Jeong J.J., Yoo S.Y., Kim D.H. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol. 2016;16:9. doi: 10.1186/s12866-016-0625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter G.A., O’Connor J.C. Brain-derived neurotrophic factor and inflammation in depression: Pathogenic partners in crime? World J. Psychiatry. 2022;12:77–97. doi: 10.5498/wjp.v12.i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bojović K., Ignjatović Ð.I., Soković Bajić S., Vojnović Milutinović D., Tomić M., Golić N., Tolinački M. Gut Microbiota Dysbiosis Associated with Altered Production of Short Chain Fatty Acids in Children with Neurodevelopmental Disorders. Front. Cell Infect. Microbiol. 2020;10:223. doi: 10.3389/fcimb.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angelucci F., Cechova K., Amlerova J., Hort J. Antibiotics, gut microbiota, and Alzheimer’s disease. J. Neuroinflamm. 2019;16:108. doi: 10.1186/s12974-019-1494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barko P.C., McMichael M.A., Swanson K.S., Williams D.A. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 2018;32:9–25. doi: 10.1111/jvim.14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carabotti M., Scirocco A., Maselli M.A., Severi C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 11.Gomaa E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek. 2020;113:2019–2040. doi: 10.1007/s10482-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 12.Bercik P., Park A.J., Sinclair D., Khoshdel A., Lu J., Huang X., Deng Y., Blennerhassett P.A., Fahnestock M., Moine D., et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto-Sanchez M.I., Hall G.B., Ghajar K., Nardelli A., Bolino C., Lau J.T., Martin F.P., Cominetti O., Welsh C., Rieder A., et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients with Irritable Bowel Syndrome. Gastroenterology. 2017;153:448–459.e448. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Lee H.J., Hong J.K., Kim J.K., Kim D.H., Jang S.W., Han S.W., Yoon I.Y. Effects of Probiotic NVP-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2021;13:2660. doi: 10.3390/nu13082660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han S.K., Kim J.K., Joo M.K., Lee K.E., Han S.W., Kim D.H. Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 Alleviate Escherichia coli-Induced depression and Gut Dysbiosis in Mice. J. Microbiol. Biotechnol. 2020;30:1222–1226. doi: 10.4014/jmb.2002.02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian P., O’Riordan K.J., Lee Y.K., Wang G., Zhao J., Zhang H., Cryan J.F., Chen W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress. 2020;12:100216. doi: 10.1016/j.ynstr.2020.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian P., Chen Y., Zhu H., Wang L., Qian X., Zou R., Zhao J., Zhang H., Qian L., Wang Q., et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022;100:233–241. doi: 10.1016/j.bbi.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Huang L., Lv X., Ze X., Ma Z., Zhang X., He R., Fan J., Zhang M., Sun B., Wang F., et al. Combined probiotics attenuate chronic unpredictable mild stress-induced depressive-like and anxiety-like behaviors in rats. Front. Psychiatry. 2022;13:990465. doi: 10.3389/fpsyt.2022.990465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slykerman R.F., Hood F., Wickens K., Thompson J.M.D., Barthow C., Murphy R., Kang J., Rowden J., Stone P., Crane J., et al. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine. 2017;24:159–165. doi: 10.1016/j.ebiom.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma X., Yoo J.W., Shin Y.J., Park H.S., Son Y.H., Kim D.H. Alleviation of Porphyromonas gingivalis or Its Extracellular Vesicles Provoked Periodontitis and Cognitive Impairment by Lactobacillus pentosus NK357 and Bifidobacterium bifidum NK391. Nutrients. 2023;15:1068. doi: 10.3390/nu15051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeung K.S., Hernandez M., Mao J.J., Haviland I., Gubili J. Herbal medicine for depression and anxiety: A systematic review with assessment of potential psycho-oncologic relevance. Phytother. Res. 2018;32:865–891. doi: 10.1002/ptr.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Lorenzo A., Nabavi S.F., Sureda A., Moghaddam A.H., Khanjani S., Arcidiaco P., Nabavi S.M., Daglia M. Antidepressive-like effects and antioxidant activity of green tea and GABA green tea in a mouse model of post-stroke depression. Mol. Nutr. Food Res. 2016;60:566–579. doi: 10.1002/mnfr.201500567. [DOI] [PubMed] [Google Scholar]
- 23.Fan X., Xiao X., Mao X., Chen D., Yu B., Wang J., Yan H. Tea bioactive components prevent carcinogenesis via anti-pathogen, anti-inflammation, and cell survival pathways. IUBMB Life. 2021;73:328–340. doi: 10.1002/iub.2445. [DOI] [PubMed] [Google Scholar]
- 24.Shen M., Yang Y., Wu Y., Zhang B., Wu H., Wang L., Tang H., Chen J. L-theanine ameliorate depressive-like behavior in a chronic unpredictable mild stress rat model via modulating the monoamine levels in limbic-cortical-striatal-pallidal-thalamic-circuit related brain regions. Phytother. Res. 2019;33:412–421. doi: 10.1002/ptr.6237. [DOI] [PubMed] [Google Scholar]
- 25.Yoo J.W., Shin Y.J., Ma X., Son Y.H., Jang H.M., Lee C.K., Kim D.H. The Alleviation of Gut Microbiota-Induced Depression and Colitis in Mice by Anti-Inflammatory Probiotics NK151, NK173, and NK175. Nutrients. 2022;14:2080. doi: 10.3390/nu14102080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joo M.K., Ma X., Yoo J.W., Shin Y.J., Kim H.J., Kim D.H. Patient-derived Enterococcus mundtii and its capsular polysaccharides cause depression through the downregulation of NF-κB-involved serotonin and BDNF expression. Microbes Infect. 2023:105116. doi: 10.1016/j.micinf.2023.105116. [DOI] [PubMed] [Google Scholar]
- 27.Lee H.J., Lee K.E., Kim J.K., Kim D.H. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Sci. Rep. 2019;9:11814. doi: 10.1038/s41598-019-48342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang H.M., Lee H.J., Jang S.E., Han M.J., Kim D.H. Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol. 2018;11:1386–1397. doi: 10.1038/s41385-018-0042-3. [DOI] [PubMed] [Google Scholar]
- 29.Lee K.E., Kim J.K., Han S.K., Lee D.Y., Lee H.J., Yim S.V., Kim D.H. The extracellular vesicle of gut microbial Paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome. 2020;8:107. doi: 10.1186/s40168-020-00881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith S.M., Vale W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehmann M.L., Brachman R.A., Listwak S.J., Herkenham M. NF-kappaB activity affects learning in aversive tasks: Possible actions via modulation of the stress axis. Brain Behav. Immun. 2010;24:1008–1017. doi: 10.1016/j.bbi.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freimer D., Yang T.T., Ho T.C., Tymofiyeva O., Leung C. The gut microbiota, HPA axis, and brain in adolescent-onset depression: Probiotics as a novel treatment. Brain Behav. Immun. Health. 2022;26:100541. doi: 10.1016/j.bbih.2022.100541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gotlib I.H., Joormann J., Minor K.L., Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol. Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peirce J.M., Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J. Neurosci. Res. 2019;97:1223–1241. doi: 10.1002/jnr.24476. [DOI] [PubMed] [Google Scholar]
- 35.Wahlestedt C., Ekman R., Widerlöv E. Neuropeptide Y (NPY) and the central nervous system: Distribution effects and possible relationship to neurological and psychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1989;13:31–54. doi: 10.1016/0278-5846(89)90003-1. [DOI] [PubMed] [Google Scholar]
- 36.Stevens C., Walz G., Singaram C., Lipman M.L., Zanker B., Muggia A., Antonioli D., Peppercorn M.A., Strom T.B. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig. Dis. Sci. 1992;37:818–826. doi: 10.1007/BF01300378. [DOI] [PubMed] [Google Scholar]
- 37.Lee D.H., Lee J.Y., Hong D.Y., Lee E.C., Park S.W., Lee M.R., Oh J.S. Neuroinflammation in Post-Traumatic Stress Disorder. Biomedicines. 2022;10:953. doi: 10.3390/biomedicines10050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L., Yao X., Ma M., Ding Y., Zhang H., He X., Song Z. Protective Effect of l-Theanine against DSS-Induced Colitis by Regulating the Lipid Metabolism and Reducing Inflammation via the NF-κB Signaling Pathway. J. Agric. Food Chem. 2021;69:14192–14203. doi: 10.1021/acs.jafc.1c05839. [DOI] [PubMed] [Google Scholar]
- 39.Jang H.M., Kim J.K., Joo M.K., Shin Y.J., Lee C.K., Kim H.J., Kim D.H. Transplantation of fecal microbiota from patients with inflammatory bowel disease and depression alters immune response and behavior in recipient mice. Sci. Rep. 2021;11:20406. doi: 10.1038/s41598-021-00088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukui H. Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm. Intest. Dis. 2016;1:135–145. doi: 10.1159/000447252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cenit M.C., Sanz Y., Codoñer-Franch P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017;23:5486–5498. doi: 10.3748/wjg.v23.i30.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jang S.E., Lim S.M., Jeong J.J., Jang H.M., Lee H.J., Han M.J., Kim D.H. Gastrointestinal inflammation by gut microbiota disturbance induces memory impairment in mice. Mucosal Immunol. 2018;11:369–379. doi: 10.1038/mi.2017.49. [DOI] [PubMed] [Google Scholar]
- 43.Peng C.H., Chiou S.H., Chen S.J., Chou Y.C., Ku H.H., Cheng C.K., Yen C.J., Tsai T.H., Chang Y.L., Kao C.L. Neuroprotection by Imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur. Neuropsychopharmacol. 2008;18:128–140. doi: 10.1016/j.euroneuro.2007.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed during the present study are available from the corresponding author.