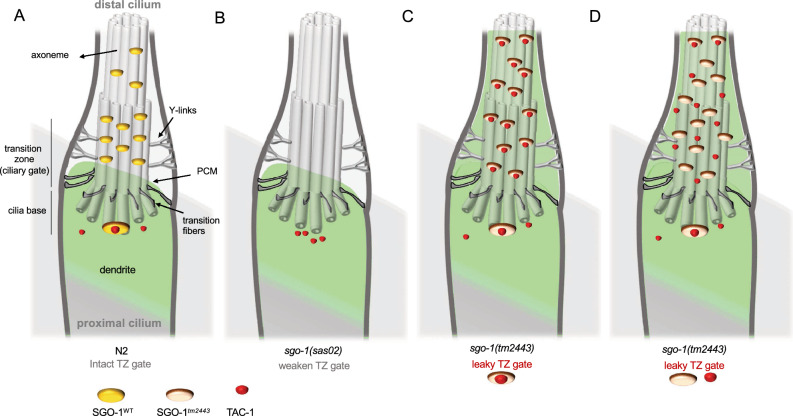
Figure 6.
(A) In wild type (N2) worms, SGO-1 binds to TAC-1 in the base of cilia. SGO-1 is also observed along the TZ and axoneme, both TAC-1-free domains in wild type cilia. In the TZ, activity of SGO-1 is likely important to sustain the impermeability of the ciliary gate (green). (B) Depletion of SGO-1 disrupts ciliary function, as attested by the behavioral defects in sgo-1(sas02) worms. While we did not observe gating defects in cilia of sas02 worms, increasing TAC-1 in these cilia (but not cilia of sas02/ + worms), disrupts the diffusion barrier. TAC-1 is widely distributed beyond the TZ gate in both sas02 and tm2443 worms, though sensory defects in the latter are markedly more severe. We interpreted these results as indicative that SGO-1-less cilia have a weakened gate, predisposed to loss of integrity, while SGO-1tm2443 enhances cilia dysfunction by enhancing TAC-1 activity in distal cilia domains of tm2443 worms. This effect of SGO-1tm2443 could be accomplished by binding and stabilizing TAC-1 in these domains (C) Conversely, TAC-1 may passively diffuse across the disrupted TZ of tm2443 mutants and remain unbound to SGO-1 in distal domains (D). PCM—periciliary membrane.